1 to 30 elements atomic mass
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight.
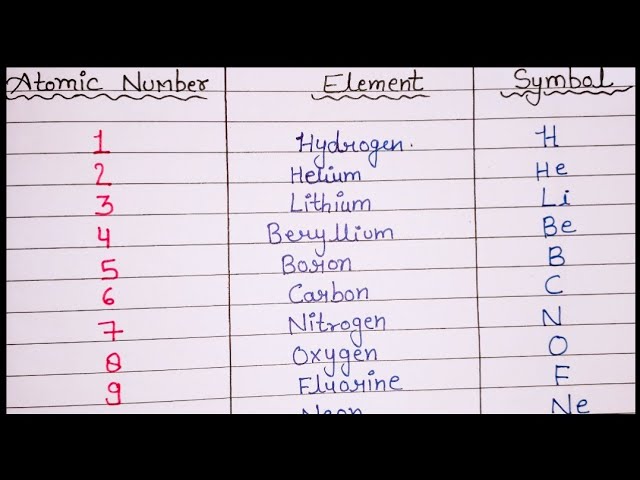
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. There are many ways to find the atomic mass of an element, but the easiest way is to look it up on the periodic table of elements. Put your understanding of this concept to test by answering a few MCQs.
1 to 30 elements atomic mass
Buka menu navigasi. Tutup saran Cari Cari. Pengaturan Pengguna. Lewati carousel. Karusel Sebelumnya. Karusel Berikutnya. Apa itu Scribd? Akademik Dokumen. Profesional Dokumen. Budaya Dokumen.
This definition provides a standard reference point for measuring atomic masses. Atomic number refers to the number of protons that are present in the nucleus of an element.
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next.
Even though atoms are very tiny pieces of matter, they have mass. Their masses are so small, however, that chemists often use a unit other than grams to express them—the atomic mass unit. Masses of other atoms are expressed with respect to the atomic mass unit. For example, the mass of an atom of 1 H is 1. Note, however, that these masses are for particular isotopes of each element. How, then, do we describe the mass of a given element? As stated above, most elements occur naturally as a mixture of two or more isotopes. For some elements, one particular isotope is much more abundant than any other isotopes. For example, naturally occurring hydrogen is nearly all hydrogen-1, and naturally occurring oxygen is nearly all oxygen
1 to 30 elements atomic mass
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements.
Noom cancellation reviews
Enhance the article with your expertise. Why was carbon selected as the reference element for calculating atomic mass? Tribology Tribology. Explore offer now. This document lists the atomic numbers, element symbols, and atomic masses of the first 30 elements. What are Monovalent Ions? Notes, Symbols Notes, Symbols. Download Adda App. But hurry up, because the offer is ending on 29th Feb! Gram Atomic and Gram Molecular Mass. Chemistry - Elements Chemistry - Elements. Jump to Page. Mough aondofa January 17, at pm. Lewati carousel. Mudita Shekhawat February 4, at am.
Atomic mass of all elements along with the rounded off values is mentioned in the chart below.
Apa itu Scribd? Login To View Results. It provides the atomic mass of each element after rounding off to the nearest whole number or half number. Periodic Law Periodic Law. Share your thoughts in the comments. Budaya Dokumen. Watch Now. Engineering Exam Experiences. Tandai sebagai konten tidak pantas. Suggest Changes. This means that one atom has an atomic mass of exactly The atomic mass of an element is the average mass of its atoms measured in atomic mass units amu, commonly known as daltons, D. This document lists the atomic numbers, element symbols, and atomic masses of the first 30 elements. Periodic Table of Elements. An atomic mass unit amu is a unit of mass used to express atomic and molecular mass.

Willingly I accept. The theme is interesting, I will take part in discussion.
The important and duly answer