10 ml of a compound containing n and o
Ref document number : Country of ref document : EP. Kind code of ref document : A1. Ref country code : DE.
We have 2 atoms of "N" on the right, so we need 2 atoms of "N" on the left. We have 6 "H" on the left, so we need 6 "H" on the right. We have 3 "O" on the right, so we need 3 "O" on the left. A mixture containing 10 mL of a nitrogen oxide and 30 ml of hydrogen reacts completely to form 10 mL of nitrogen. What is the formula for the nitrogen oxide? Ernest Z.
10 ml of a compound containing n and o
What is the empirical formula of this compound? Volume of 1. Molecular formula of the compound is. The resultant solution has:. It is :. The volume of the gas after explosion was 90 mL. On treatment with KOH solution, a further contraction of 20 mL in volume was observed. The vapour density of the compound is All volume measurements were made under the same condition. The molecular formula of the compound is. Volume of the gas after explosion was 90 mL. On treatment with KOH solution,further contraction of 20 mL in volume was observed. If 30mL of H 2 and 20mL of O 2 react to form form water, what is left at the end of the reaction? The molarity of the solution containing 2.
Start Your Infinity Experience. When the polymer is a non-conjugated high polymer, the above boron-containing organic compound is on the side chain of the high polymer.
If Te is the temperature at equilibrium, the reaction would be spontaneous when. A solution containing 2. The chloride ions obtained is solution was treated with excess of AgNO 3 to give 4. The formula of the complex is At. The excess of the acid required 15 mL of 0. The percentage of nitrogen in the compound is.
Sign in Open App. Molecular formula of compound if both reactants react completely, is. Correct answer is option 'C'. Can you explain this answer? Verified Answer. Most Upvoted Answer. The question is incomplete as it does not specify the concentration or state of the compound. Please provide more information to answer the question accurately. View all answers. Explore Courses for Class 11 exam.
10 ml of a compound containing n and o
We have 2 atoms of "N" on the right, so we need 2 atoms of "N" on the left. We have 6 "H" on the left, so we need 6 "H" on the right. We have 3 "O" on the right, so we need 3 "O" on the left. A mixture containing 10 mL of a nitrogen oxide and 30 ml of hydrogen reacts completely to form 10 mL of nitrogen. What is the formula for the nitrogen oxide? Ernest Z.
Control app porn game
When 20ml of mixture of O 2 and O 3 is heated the volume becomes 29m If the radius of the cation is pm, the radius of the anion is pm pm pm pm. The host material, matrix material, Host material, and Matrix material have the same meaning and are interchangeable. Polycyclic aromatic compounds and organic electroluminescent devices using the same. Synthesis, characterization, and biological target prediction of novel 1,3-dithiolo[4,5- b ]quinoxaline and thiazolo[4,5- b ]quinoxaline derivatives. Instant help, 24x7. Synthesis, characterization, and antimicrobial activity of a novel trisazo dye from 3-amino-4H-thieno[3,4-c][1]benzopyranone. Printing, lithography, flexographic printing, rotary printing, spraying, brushing or pad printing or slit-type extrusion coating. Thereby, the boron-containing organic compound can be used for a printed OLED. Get App. The total number of neutrons present in 10g D 2 O D "is" 2 H are. Boron-containing organic compound and applications thereof, organic mixture, and organic electronic device. All authors have given approval to the final version of the paper.
What is the empirical formula of this compound? Volume of 1. Molecular formula of the compound is.
Figure 1. Welcome Back. Essay review. Go Premium and unlock limitless education potential beyond daily practice limits! See all questions in Determining Formula. Question 4. Polycyclic aromatic compounds and organic electroluminescent devices using the same. In summary, a new poly azo dye containing four diazo bridges and incorporating a dibenzobarrelene backbone and four phenyl substituents has been synthesized by coupling the N -aryl succinimid fused to dibenzobarrelene with phenyl diazonium hydrogenosulfate. The molarity of the solution containing 2. In particular, it is described by the substituents L1 to L6 which compounds are particularly suitable for which functions. Views: 5, Evaluation of the effect of azo group on the biological activity of 1- 4-methylphenylazo naphthol. An excess of 1. Which one of the following has an optical isomer? When 20ml of mixture of O 2 and O 3 is heated the volume becomes 29m

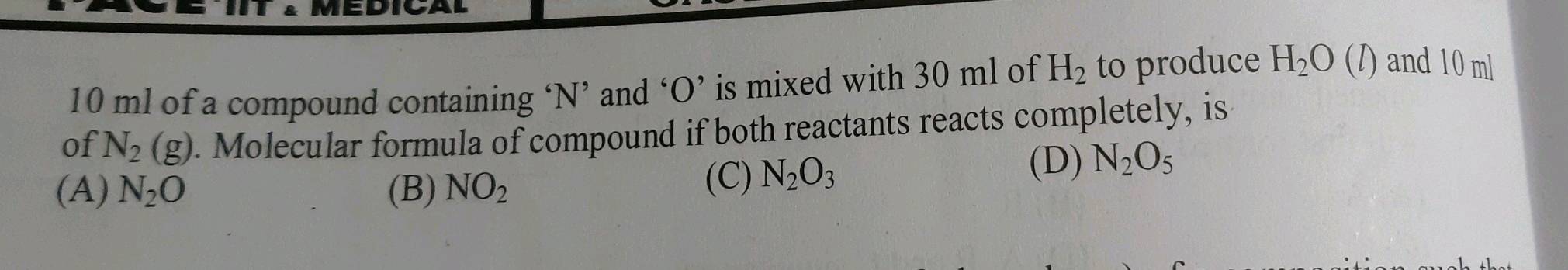
And variants are possible still?
I join. So happens. Let's discuss this question.