2agcl
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl, 2agcl. 2agcl white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, 2agcl, silver chloride converts to silver and chlorinewhich is signaled by grey to black or purplish coloration in some samples.
Then, lookup atomic weights for each element in periodic table : Ba: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
2agcl
.
Silver chloride does not react with nitric acid, but instead reacts with sulfuric acid to produce silver sulfate, 2agcl.
.
Silver chloride is a white crystalline chemical compound with the formula AgCl. Silver chloride in the test tube quickly turns purplish, especially in a sunny laboratory because the silver chloride is split up into silver and chlorine. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs. Silver chloride is an example of a well-known salt stain used to impart an amber colour to the glass. AgCl has several disinfectant and antiseptic properties and is also used in the treatment of mercury poisoning.
2agcl
Then, lookup atomic weights for each element in periodic table : Ag: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
Good luck in pashto
Silver compounds. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. CuCl CuCl 2. CRC Press. Retrieved 28 July Example: calculating molar mass Let's calculate the molar mass of carbon dioxide CO 2 : Carbon C has an atomic mass of about AgCl quickly darkens on exposure to light by disintegrating into elemental chlorine and metallic silver. In , Schulze made a paste of silver nitrate and chalk, placed the mixture in a glass bottle, and wrapped the bottle in This conversion is a common test for the presence of chloride in solution. Retrieved Then at 11 GPa, it undergoes another phase change to an orthorhombic TlI phase. MnCl 2 MnCl 3. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Studies in Conservation.
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine , which is signaled by grey to black or purplish coloration in some samples.
Then at 11 GPa, it undergoes another phase change to an orthorhombic TlI phase. But the first person to use this property to produce a photographic image was German physicist Johann Heinrich Schulze. Bibcode : Mate For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. AmCl 2 AmCl 3. It is usually the internal reference electrode in pH meters and it is often used as a reference in reduction potential measurements. Retrieved 25 February Hull; D. PMC In one of the most famous reactions in chemistry, the addition of colorless aqueous silver nitrate to an equally colorless solution of sodium chloride produces an opaque white precipitate of AgCl: [14]. Space group. Coordination geometry. Archived from the original PDF on December 3, Article Talk. Interactive image.

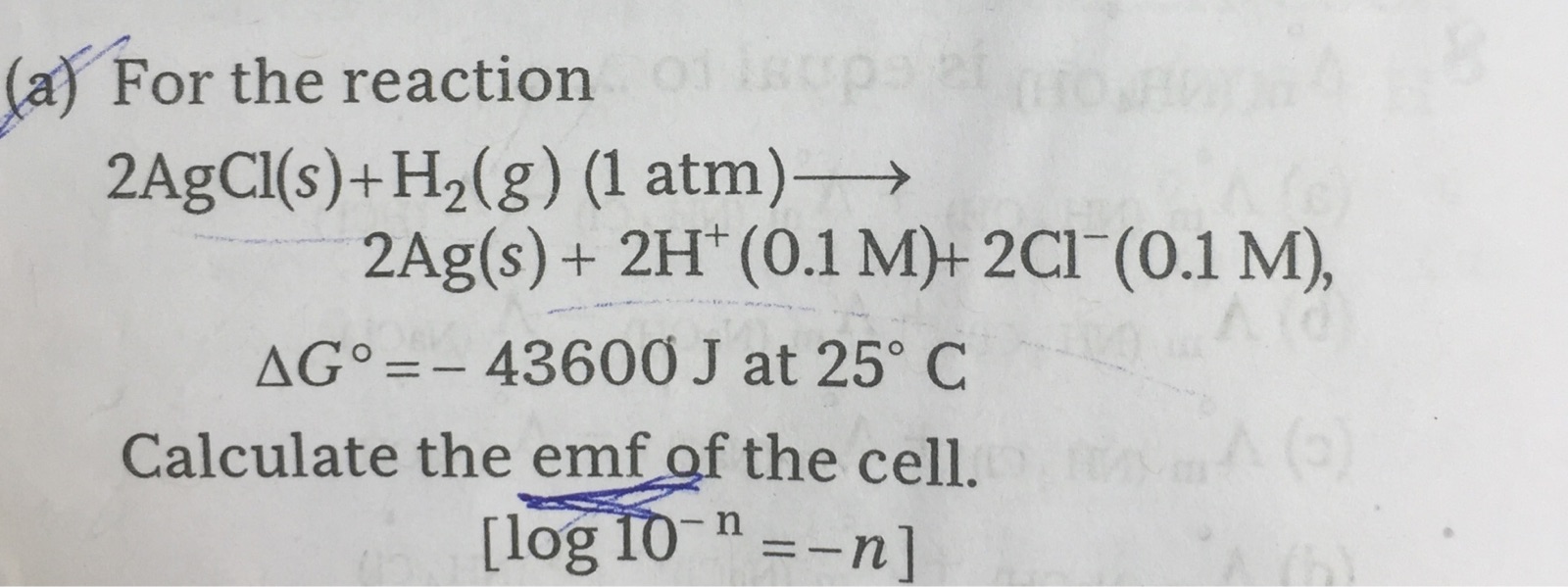
I have found the answer to your question in google.com