4.0 g of a gas occupies
A mass of diatomic gas at a pressure of 2 atm is compressed adiabatically so that its temperature rise from 27 o C to o C. The pressure of the gas is final state is. Change in entropy.
The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. So, if you are given these values for temperature and pressure, the volume occupied by any number of moles of an ideal gas can be easily derived from knowing that 1 mole occupies Let's say you were given a temperature of K and a pressure of 2. Since molar volume refers to the volume occupied by 1 mole, you'd get. This is how much volume 1 mole occupies at K and 2. It becomes clear that the volume occupied by any number of moles at these conditions can be easily determined:. As a conclusion, knowing a gas' molar volume at a certain temperature and a certain pressure can simplify the calculation of the volume occupied by any number of moles of that respective gas.
4.0 g of a gas occupies
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - Teaches : Physics, Mathematics. Total classes on Filo by this tutor - 1, Views: 5, Connect with our Physics tutors online and get step by step solution of this question. Are you ready to take control of your learning? Class Specific heat capacity. The specific he. Solving time: 3 mins. Views: 5, students. Updated on: Nov 10, Was this solution helpful? Filo tutor solutions 5 Learn from their 1-to-1 discussion with Filo tutors.
Question Type. Essay review. How many hydrogen molecules are in 2.
Q: Two identical balloons are inflated and charged in the same manner. They are tied by threads and…. Q: circuit containing a capacitor and an inductor in series has a resonant frequency of kHz. Q: What methods of heat transfer are involved when heat flows through a glass windowpane? Select all….
Avogadro's law states that, at the same temperature and pressure, equal volumes of all gases have the same number of molecules. The volume increases as the number of moles increases. It does not depend on the sizes or the masses of the molecules. One mole of an ideal gas occupies Thus, its molar volume at STP is
4.0 g of a gas occupies
This action cannot be undone. This will permanently delete All Practiced Questions. Only bookmarked questions of selected question set or default questions are shown here. Click Here to view all bookmarked questions of the chapter. The specific heat capacity of the gas at constant volume is 5.
Contacto con gays
Is the volume of a gas always the volume of its container? Assuming the Snapsolve any problem by taking a picture. What is the molecular mass of this gas? AP Police Constable. The completed exercise should be submitted online via WebLearn. Human can hear sound in range of:. AOC Material Assistant. What is the mass of 7. Uttarakhand D. If the hot asphalt Previous Doubts. Maharashtra Zilla Parishad Rigman.
What is the molar mass of the gas? Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators.
IOCL Apprentice. Question e96d5. Q: Two identical balloons are inflated and charged in the same manner. This is beneficial…. Calculate the molar specific heat of monoatomic gas at constant volume. Was the final answer of the question wrong? TN TRB. Slope of distance-time graph gives:. During an isothermal expansion, a confined ideal gas does - J of work against its surroundings. The mass of each of the two blocks is 1.

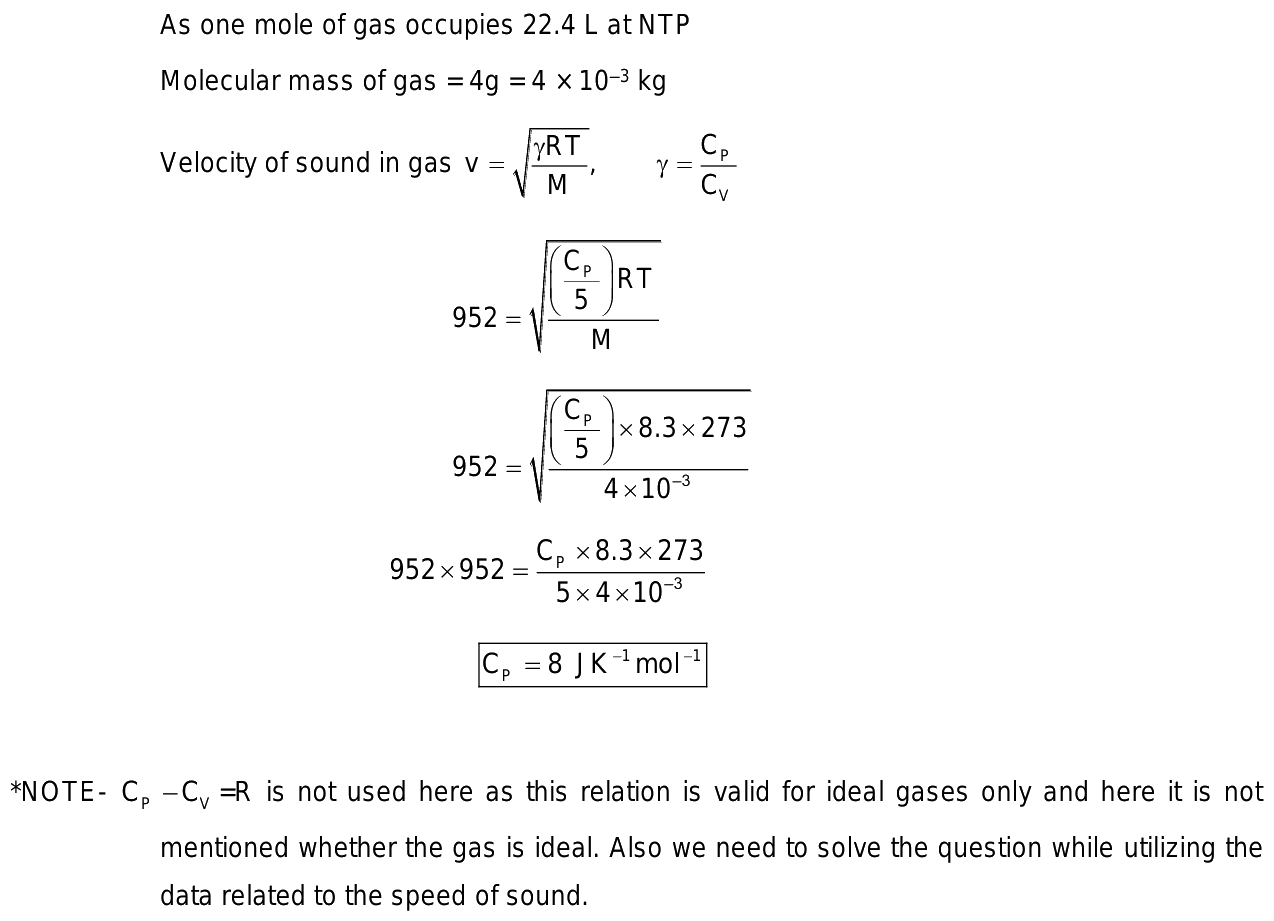
0 thoughts on “4.0 g of a gas occupies”