Acetylene lewis structure
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms.
Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a colorless, flammable gas with the chemical formula C2H2. This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. The hottest and most efficient fuel gas, acetylene, provides high productivity levels thanks to good localized heating with minimal thermal waste. It also requires the least amount of oxygen to ensure complete combustion. This flammable, colorless gas is lighter than air, so it does not accumulate at low levels, where it could cause a potential hazard. It is generally supplied dissolved in acetone or DMF.
Acetylene lewis structure
.
In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
.
Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond. As acetylene is reactive, unstable, and lighter than the air, the gas is highly flammable and leads to an explosion. Irrespective of being toxic, acetylene is used for welding purposes as it is flammable. To human beings, this compound is no less than an element of risk as to the existence of it in the atmosphere reduces the level of oxygen.
Acetylene lewis structure
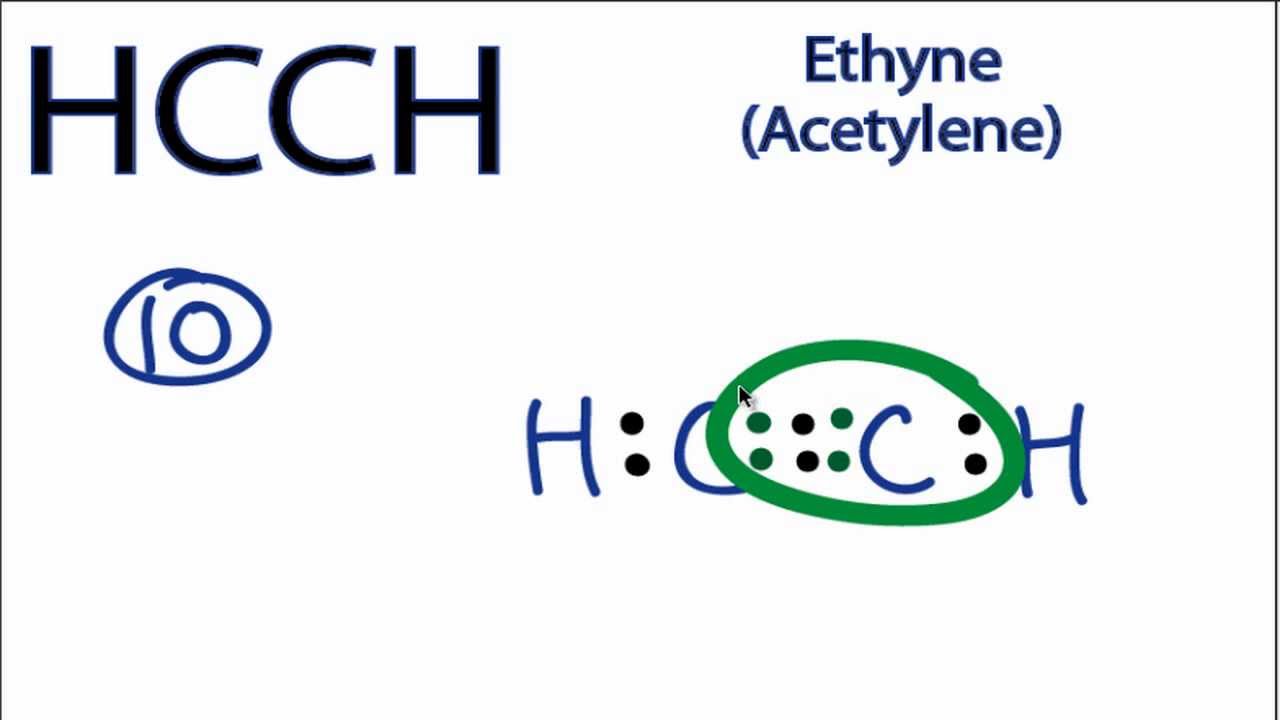
The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two hydrogen atoms. The molecule has two triple bonds, which means each carbon atom is connected to the other with a triple bond.
Ninja foodi max 14-in-1
Lewis Structure Lewis Structure of any molecule helps to know the arrangement of all atoms, their valence electrons, and the bond formation in the molecule. C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. It is generally supplied dissolved in acetone or DMF. But, there are no charges on hydrogen atoms. To be the center atom, ability of having greater valance and being a electropositive element are important facts. If there are charges on atoms and if those charges can be reduced by converting lone pairs to bonds, we should do that to obtain the best stable lewis structure. There is a double bond between two carbon atoms in acetylene. Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water. This flammable, colorless gas is lighter than air, so it does not accumulate at low levels, where it could cause a potential hazard. Now, we are going to draw that C 2 H 4 lewis structure step by step. What is Phenoxyacetic acid used for? You may like Red-to-black electrochromism of 4, There are no lone pairs on carbon and hydrogen atom in their valence shells because all valence electrons are contributed to make bonds.
It is a hydrocarbon and the simplest alkyne. It is unstable in its pure form and thus is usually handled as a solution.
Lewis Structure Lewis Structure of any molecule helps to know the arrangement of all atoms, their valence electrons, and the bond formation in the molecule. Introduce Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. Related articles Related Qustion. There are only two sigma bonds around a carbon atom. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step. There is a double bond between two carbon atoms in acetylene. What is the Lewis structure for acetylene? There are no lone pairs on carbon or hydrogen atoms. The hottest and most efficient fuel gas, acetylene, provides high productivity levels thanks to good localized heating with minimal thermal waste. Because C 2 H 2 molecule is a simple molecule, those all steps may not be used. In the above structure, there ar charges on both carbon atoms. In this structure, in addition to a complete duplet of the outer H-atoms, the two C-atoms at the center also have a complete octet with 1 mutually shared triple covalent bond and a C-H single bond at each terminal. Now, we are going to draw that C 2 H 4 lewis structure step by step. Dec 19, The charges in the Acetylene molecule are evenly distributed, and there are no positively charged or negatively charged areas in the molecule.

I congratulate, very good idea
I consider, that you are not right. I can defend the position. Write to me in PM.
I consider, that you are mistaken. I can defend the position. Write to me in PM.