Al cuso4 reaction
In association with Al cuso4 reaction Foundation. Try this class practical or demonstration to illustrate the displacement of copper from copper II sulfate using aluminium foil. In this experiment, students add aluminium cooking foil to copper II sulfate solution and observe no reaction. They then add and dissolve sodium chloride, producing a vigorous displacement reaction which illustrates the reactivity of aluminium.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method.
Al cuso4 reaction
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges. This method separates the reaction into two half-reactions — one for oxidation and one for reduction.
The limiting reagent row will be highlighted in pink. The equation is balanced.
.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced.
Al cuso4 reaction
In association with Nuffield Foundation. Try this class practical or demonstration to illustrate the displacement of copper from copper II sulfate using aluminium foil. In this experiment, students add aluminium cooking foil to copper II sulfate solution and observe no reaction.
Chienbaise femme
Experiment Practical potions microscale 11—14 years By Kirsty Patterson Observe chemical changes in this microscale experiment with a spooky twist. Additional information This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. Chemistry tools. In many cases a complete equation will be suggested. By Kirsty Patterson. Use a related experiment from our Exhibition Chemistry series to demonstrate the reactivity of aluminium using hydrochloric acid and mercury. This allows reaction with the copper II sulfate. By Kristy Turner. Experiment Antibacterial properties of the halogens 14—18 years By Kristy Turner Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective. Balancing with inspection or trial and error method This is the most straightforward method.
.
Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables. This allows reaction with the copper II sulfate. Best For: Redox reactions where electron transfer occurs. It shows the reactants substances that start a reaction and products substances formed by the reaction. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. Site powered by Webvision Cloud. Best for: Equations that are more complex and not easily balanced by inspection. By Kristy Turner. Temperature change. Let's balance this equation using the algebraic method. Resource Paracetamol book The extraction and purification of paracetamol from tablets How pure is paracetamol? The equipment required for illustrating the reaction between copper II sulfate and aluminium, before sodium chloride is added to disrupt the oxide layer on the aluminium foil. In this activity you investigate the purity and identity of your laboratory prepared samples of nitrophenol or paracetamol using thin-layer chromatography. How pure is paracetamol?

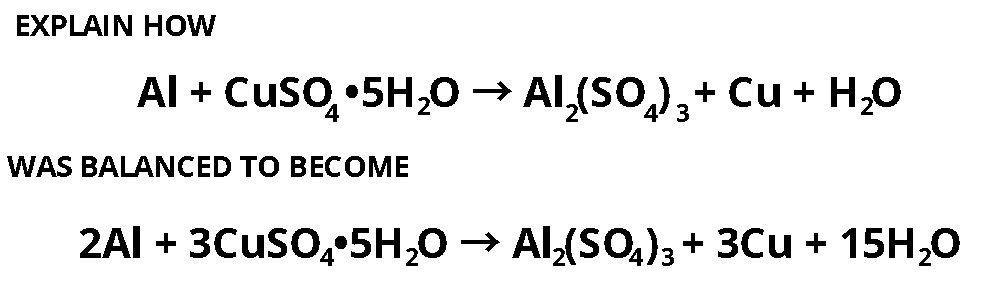
I have forgotten to remind you.