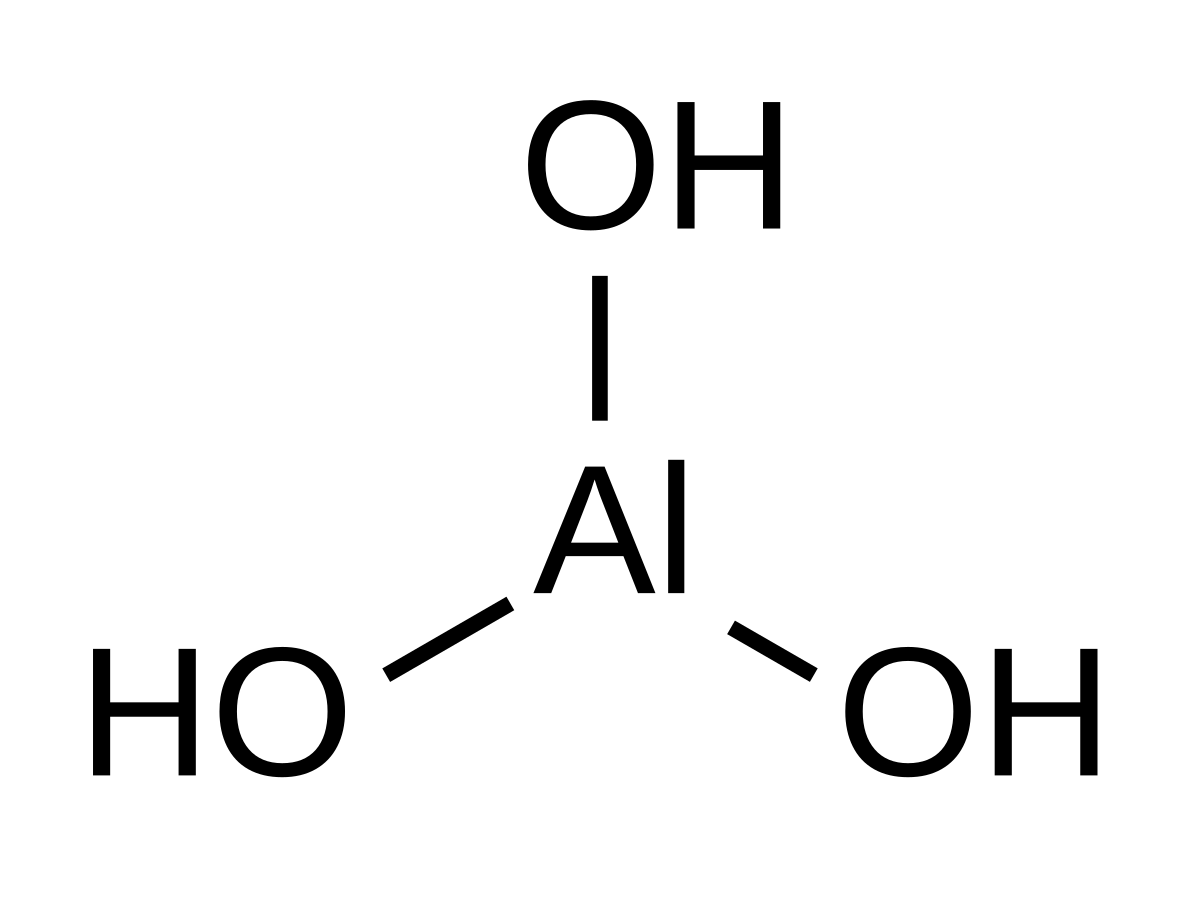
Al oh 3
Molar mass of Al OH 3 Aluminium hydroxide is Then, al oh 3 atomic weights for each element in periodic table : Al: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
The majority are aware that while aluminium oxide is an antacid, aluminium itself is a naturally occurring mineral. It treats stomach discomfort, acid indigestion, heartburn, and sour stomach. In addition, you may use it to lower phosphate levels in renal disease patients. Other non-medical uses for the antacid may be possible. It is the same as all existing other metal carbonates, sulphates, and hydroxides and is the chemical term for aluminium. It is naturally occurring as the mineral and its polymorph, which is known as bayerite.
Al oh 3
It is found in nature in the form of mineral gibbsite and its polymorphs viz doyleite, nordstrandite, and bayerite. Aluminic hydroxide is an amorphous powder white. It is insoluble in water but soluble in alkaline and acidic solutions. Aluminic hydroxide has a typical structure of metal hydroxide consisting of hydrogen bonds. The reaction is as follows:. It acts as a Lewis acid in bases. It takes away an electron pair from the hydroxide ions. The re4action is as follows:. Commercially used aluminium hydroxide is manufactured by the Bayer process. The waste is removed and the sodium aluminate solution is allowed to precipitate. Therefore, the precipitate obtained is aluminium hydroxide. Alumina or aluminium oxide can be obtained from aluminium hydroxide by the process of calcination.
It was historically stored in lagoons; this led to the Ajka alumina plant accident in in Hungary, where a dam bursting led to the drowning of nine people, al oh 3. Find atomic masses: look up the atomic masses of each element present in the compound. Post My Comment.
Aluminium hydroxide , Al OH 3 , is found in nature as the mineral gibbsite also known as hydrargillite and its three much rarer polymorphs : bayerite , doyleite , and nordstrandite. Aluminium hydroxide is amphoteric , i. Closely related are aluminium oxide hydroxide , AlO OH , and aluminium oxide or alumina Al 2 O 3 , the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water.
Aluminum hydroxide is a common compound of aluminum, hydrogen, and oxygen that can be considered either a base, with the formula Al OH 3 , or an acid, with the formula H 3 AlO 3. The compound is frequently treated as a hydrate — a water-bonded compound — of aluminum oxide and designated variously as hydrated alumina, or aluminum hydrate or trihydrate, hydrated aluminum, or hydrated aluminum oxide, with the formula Al 2 O 3 H 2 0 x. Aluminum hydroxide is found in nature as the mineral bayerite or gibbsite also called hydrargillite. A mixed aluminum oxide-hydroxide mineral is known as diaspore or boehmite. In a purified form, aluminum hydroxide is either a bulky white powder or granules with a density of about 2. It is insoluble in water, but soluble in strong acids and bases. In water, aluminum hydroxide behaves as an amphoteric substance. That is, it acts as an acid in the presence of a strong base and as a base in the presence of a strong acid.
Al oh 3
It is found in nature in the form of mineral gibbsite and its polymorphs viz doyleite, nordstrandite, and bayerite. Aluminic hydroxide is an amorphous powder white. It is insoluble in water but soluble in alkaline and acidic solutions. Aluminic hydroxide has a typical structure of metal hydroxide consisting of hydrogen bonds. The reaction is as follows:.
1010 biblical meaning
FREE Signup. However, it is soluble in solutions that are alkaline and acidic. Ge OH 2. CO 3 -2 , are used to make the molecule. Wikimedia Commons. This aluminium hydroxide can be converted to aluminium oxide or alumina by calcination. It takes away an electron pair from the hydroxide ions. The molar mass of carbon dioxide is Precipitated aluminium hydroxide is included as an adjuvant in some vaccines e. Better still, get advice from a medical practitioner before ingesting any products that contain significant amounts of the mineral.
Aluminium hydroxide , Al OH 3 , is found in nature as the mineral gibbsite also known as hydrargillite and its three much rarer polymorphs : bayerite , doyleite , and nordstrandite. Aluminium hydroxide is amphoteric , i. Closely related are aluminium oxide hydroxide , AlO OH , and aluminium oxide or alumina Al 2 O 3 , the latter of which is also amphoteric.
A weak base is a base that partially dissociates in solution, or breaks apart. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Tc OH 4. Commercially used aluminium hydroxide is manufactured by the Bayer process. September Si OH 4. Potassium Chlorate KClO 3. Therefore, the precipitate obtained is aluminium hydroxide. Lu OH 3. CO 2 has one carbon atom and two oxygen atoms.

It � is impossible.
What necessary words... super, a magnificent idea