An element crystallizes in bcc structure
Lithium crystallizes in a body centred cubic lattice.
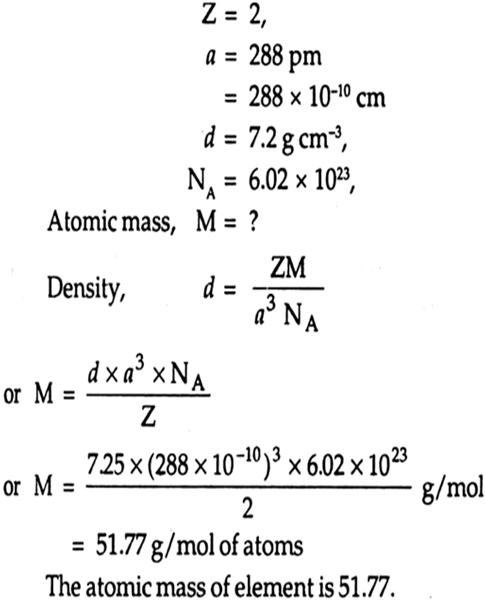
As element cystallises in BCC structure. The edge length of its unit cell is pm. It the density of the crystals is 7. Aluminium has fcc structure. The length of the unit cell is pm.
An element crystallizes in bcc structure
An element crystallizes in a b. The density of the element is 7. How many atoms are present in g of the element? CBSE Class 12 physics board exam today; values of physical constants, weightage. Dont't have an account? Register Now. Colleges Colleges Accepting B. Quick links BTech M. Computer Application and IT Change. Pharmacy Change.
Analysis shows that an oxide ore of nickel has formula Ni 0. JEE Main Syllabus. Mathematics Syllabus.
Courses for Kids. Free study material. Offline Centres. Talk to our experts An element crystallizes in bcc structure.
Most solids form with a regular arrangement of their particles because the overall attractive interactions between particles are maximized, and the total intermolecular energy is minimized, when the particles pack in the most efficient manner. The regular arrangement at an atomic level is often reflected at a macroscopic level. In this module, we will explore some of the details about the structures of metallic and ionic crystalline solids, and learn how these structures are determined experimentally. We will begin our discussion of crystalline solids by considering elemental metals, which are relatively simple because each contains only one type of atom. A pure metal is a crystalline solid with metal atoms packed closely together in a repeating pattern. Some of the properties of metals in general, such as their malleability and ductility, are largely due to having identical atoms arranged in a regular pattern. The different properties of one metal compared to another partially depend on the sizes of their atoms and the specifics of their spatial arrangements.
An element crystallizes in bcc structure
Let us discuss these Principal Metallic Crystal Structures in detail. A unit cell is a building block of the Crystal structure. The crystal structure is made of atoms. A crystal lattice is made of points. The HCP structure is a denser modification of the simple hexagonal crystal structure. Most metals crystallize in these dense-packed structures because energy is released as the atoms come closer together and bond more tightly with each other. Thus, the densely packed structures are in lower and more stable energy arrangements. The extremely small size of the unit cells of crystalline metals that are shown below should be emphasized. The cube side of the unit cell of body-centered cubic iron, for example, room temperature is equal to 0. Let us now examine in detail the arrangement of the atoms in the three principal crystal structure unit cells.
Mcalisters near me
Vilume occupied for a cubic close packed lattice of sphere is Video Solution. Physics Syllabus. Courses for Kids. The edge length of its unit cell is pm. Competition Change. Home School An element crystallizes in a b. Create Your Account Name. Practice Materials. I am already a member. Welcome Back : To keep connected with us please login with your personal information by phone. How many atoms are present in g of the element?
The Body-Centered Cubic BCC crystal structure is one of the most common ways for atoms to arrange themselves in metals. The BCC crystal structure is based on the Bravais lattice of the same name, with 1 atom per lattice point at each corner of the cube and the center of the cube.
Study Abroad Change. Ask Now. An element crystallises in a b. Difference Between. Vilume occupied for a cubic close packed lattice of sphere is Physics Syllabus. JEE students also check. If diamond crystallizes in fcc form with edge length 'a' , find out a number of next nearest neighbours in diamond lattice b distance between the next nearest neighbours. Correction Window. Chemistry Mock Test. The density of crystal is 3. View Solution. For the body center atoms, the contribution of that atom is a total of a total atom. In how many years will it become 16 times itself? Important Dates.

I do not see in it sense.
The authoritative point of view, it is tempting
Yes, I understand you. In it something is also thought excellent, I support.