Are ionic bonds stronger than covalent bonds
Post by Jessica Castellanos » Tue Nov 19, am. Post by jisulee1C » Tue Nov 19, am. Post by joshtully » Mon Oct 26, am. Post by isha dis3d » Wed Oct 28, pm.
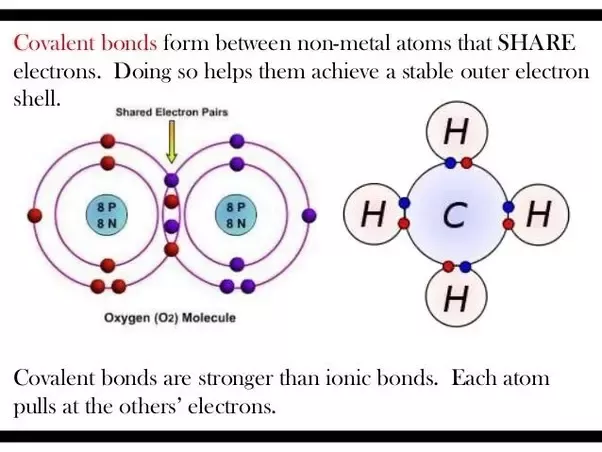
Byju's Answer. Which bond is stronger- ionic or covalent? Open in App. Chemical bonds can be either formed by sharing of electrons or by transfer of electrons, by which the bonding atoms attain an octet[or duplet] state. The bonds formed by sharing of electrons between the bonding atoms are known as covalent bonds. The bonds formed by the transfer of electrons are known as ionic bonds. Generally, ionic bonds are stronger than covalent bonds.
Are ionic bonds stronger than covalent bonds
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy; the stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. Breaking a bond always require energy to be added to the molecule. Correspondingly, making a bond always releases energy. Molecules with three or more atoms have two or more bonds. The sum of all bond energies in such a molecule is equal to the standard enthalpy change for the endothermic reaction that breaks all the bonds in the molecule. For example, the sum of the four C—H bond energies in CH 4 , kJ, is equal to the standard enthalpy change of the reaction:. The strength of a bond between two atoms increases as the number of electron pairs in the bond increases. Generally, as the bond strength increases, the bond length decreases. Thus, we find that triple bonds are stronger and shorter than double bonds between the same two atoms; likewise, double bonds are stronger and shorter than single bonds between the same two atoms. When one atom bonds to various atoms in a group, the bond strength typically decreases as we move down the group.
Post by Cynthia Gong 1L » Tue Nov 19, am ionic bonds are stronger because the molecules will form a tightly knit crystal lattice structure that is extremely strong.
Atoms separated by a great distance cannot link; rather, they must come close enough for the electrons in their valence shells to interact. But do atoms ever actually touch one another? Most physicists would say no, because the negatively charged electrons in their valence shells repel one another. No force within the human body—or anywhere in the natural world—is strong enough to overcome this electrical repulsion. So when you read about atoms linking together or colliding, bear in mind that the atoms are not merging in a physical sense. Instead, atoms link by forming a chemical bond.
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy; the stronger a bond, the greater the energy required to break it. The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy.
Are ionic bonds stronger than covalent bonds
In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy see Figure 7. The stronger a bond, the greater the energy required to break it.
Can i get bds with 250 marks in neet
Post by Adrienne Yuh 2B » Sat Oct 17, am In terms of chemistry, ionic bonds are stronger than covalent bonds! In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. Post by Sameer Chowdhury 3C » Sun Nov 01, pm I believe in order of strength at the molecular level it goes, from strongest to weakest: ionic, covalent, polar, Van der Waals forces. The first structure shows a carbon atom with a lone pair of electrons triple bonded to an oxygen with a lone pair of electrons. Post by Sophia Kalanski 1A » Sat Oct 31, pm ionic bonds are usually stronger because they are causing a transfer in electrons rather than a quick sharing of them. The enthalpy of a reaction can be estimated based on the energy input required to break bonds and the energy released when new bonds are formed. Oils are nonpolar, and are repelled by water. When all other parameters are kept constant, doubling the charge of both the cation and anion quadruples the lattice energy. In covalent bonds, the participating atoms do not lose or gain electrons, but rather share them. The bond energy is obtained from a table and will depend on whether the particular bond is a single, double, or triple bond. The oxygen atom is also single bonded to a hydrogen atom. Thus, a unit of water, or H 2 O, is a compound, as is a single molecule of the gas methane, or CH 4. No force within the human body—or anywhere in the natural world—is strong enough to overcome this electrical repulsion. These ions combine to produce solid cesium fluoride.
It is essential to remember that energy must be added to break chemical bonds an endothermic process , whereas forming chemical bonds releases energy an exothermic process. Stable molecules exist because covalent bonds hold the atoms together.
But when an atom participates in a chemical reaction that results in the donation or acceptance of one or more electrons, the atom will then become positively or negatively charged. For example, the sum of the four C—H bond energies in CH 4 , kJ, is equal to the standard enthalpy change of the reaction: A reaction is shown with Lewis structures. Skip to content The Chemical Level of Organization. Post by joshtully » Mon Oct 26, am Ionic bonds are stronger than covalent bonds. Why or why not? Thus, if you are looking up lattice energies in another reference, be certain to check which definition is being used. I think ionic bonds are stronger, especially as the electronegativity difference between two bonded atoms increases and as the ionic character of the bond increases. Chapter Review Each moment of life, atoms of oxygen, carbon, hydrogen, and the other elements of the human body are making and breaking chemical bonds. Because of the close sharing of pairs of electrons one electron from each of two atoms , covalent bonds are stronger than ionic bonds. Since increased ionic character means the bond is less covalent, it would mean that ionic is stronger. This can happen either by gaining electrons to fill a shell that is more than half-full, or by giving away electrons to empty a shell than is less than half-full, thereby leaving the next smaller electron shell as the new, full, valence shell. Covalent bonds are formed from the sharing of electrons between nuclei and, ionic bonds are formed from mutual attractions between oppositely charged ions. Post by isha dis3d » Wed Oct 28, pm Ionic bonds are stronger than covalent bonds because the electronegativity difference between the two elements is much greater than that of two elements in a covalent bond. Calculations of this type will also tell us whether a reaction is exothermic or endothermic.

You are not right. I suggest it to discuss. Write to me in PM, we will communicate.
Excuse, that I can not participate now in discussion - there is no free time. I will be released - I will necessarily express the opinion on this question.
Certainly. So happens.