Arsenic molar mass
Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties.
Molar mass of As 4 O 6 is Then, lookup atomic weights for each element in periodic table : As: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Arsenic molar mass
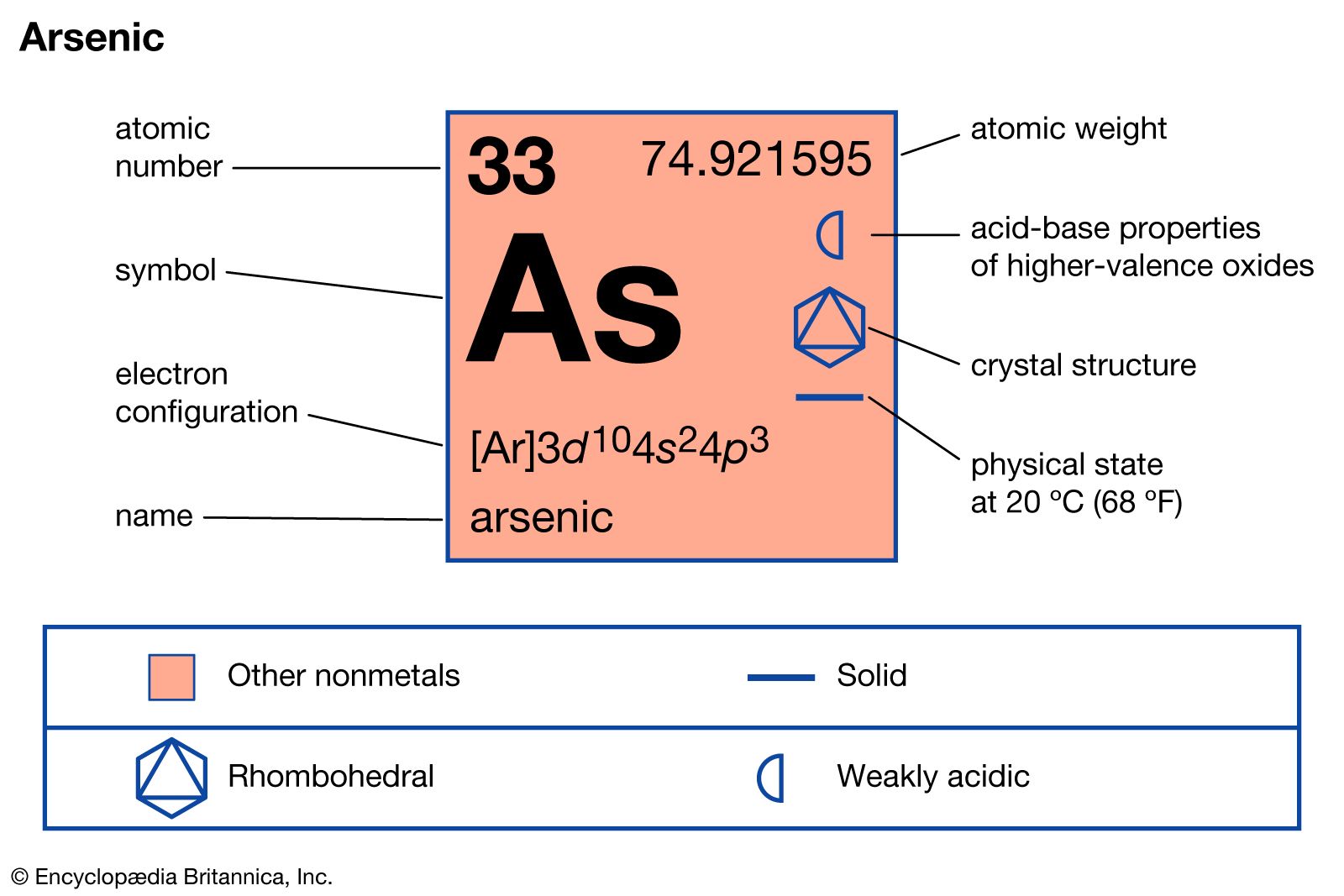
Arsenic is a chemical element ; it has symbol As and atomic number Arsenic occurs in many minerals, usually in combination with sulfur and metals , but also as a pure elemental crystal. Arsenic is a notoriously toxic metalloid. It has various allotropes , but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead for example, in car batteries and ammunition. Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III—V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides , treated wood products, herbicides , and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in rats, hamsters, goats, chickens, and presumably other species. A role in human metabolism is not known. Arsenic contamination of groundwater is a problem that affects millions of people across the world. The United States ' Environmental Protection Agency states that all forms of arsenic are a serious risk to human health. The three most common arsenic allotropes are grey, yellow, and black arsenic, with grey being the most common.
Arsenic molar mass is estimated that approximately 57 million people in the Bengal basin are drinking groundwater with arsenic concentrations elevated above the World Health Organization 's standard of 10 parts per billion ppb.
Molar mass of Arsenic As is Then, lookup atomic weights for each element in periodic table : As: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
Arsenic is a chemical element ; it has symbol As and atomic number Arsenic occurs in many minerals, usually in combination with sulfur and metals , but also as a pure elemental crystal. Arsenic is a notoriously toxic metalloid. It has various allotropes , but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead for example, in car batteries and ammunition. Arsenic is a common n-type dopant in semiconductor electronic devices.
Arsenic molar mass
Molar mass of Arsenic As is Then, lookup atomic weights for each element in periodic table : As: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
Ford ranger 2023 price philippines monthly installment
Retrieved 29 June See how this site uses Cookies. Where the element is most commonly found in nature, and how it is sourced commercially. Archived from the original on 21 August A higher recycling rate may reduce risk to supply. The main factors are pH and the redox potential. Arsenic is neither a metal nor a non-metal but instead joins a select but rather ill defined group of elements called the metalloids. Molar mass of As 4 O 6 is Na 3 As. Poisoning was some times diagnosed on the basis of a victim's garlic breath. Ground Water. Protocols for safe disposal of CCA lumber are not consistent throughout the world. GHS labelling : []. It was so common that I'd reckon 90 per cent of the horses had arsenic in their system.
Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Inorganic arsenic compounds are found in soils, sediments, and groundwater. These compounds occur either naturally or as a result of mining, ore smelting, and industrial use of arsenic.
The primary use of arsenic is in alloys of lead for example, in car batteries and ammunition. Synthetic arsenates include Scheele's Green cupric hydrogen arsenate, acidic copper arsenate , calcium arsenate , and lead hydrogen arsenate. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Enter a chemical formula to calculate its molar mass and elemental composition:. Food and Drug Administration set the "level of concern" for inorganic arsenic in apple and pear juices at 23 ppb, based on non-carcinogenic effects, and began blocking importation of products in excess of this level; it also required recalls for non-conforming domestic products. Retrieved 23 May Arsenic is a chemical element ; it has symbol As and atomic number Pharmacy in History. Fred Campbell 11 August The percentage of the world reserves located in the country with the largest reserves.

0 thoughts on “Arsenic molar mass”