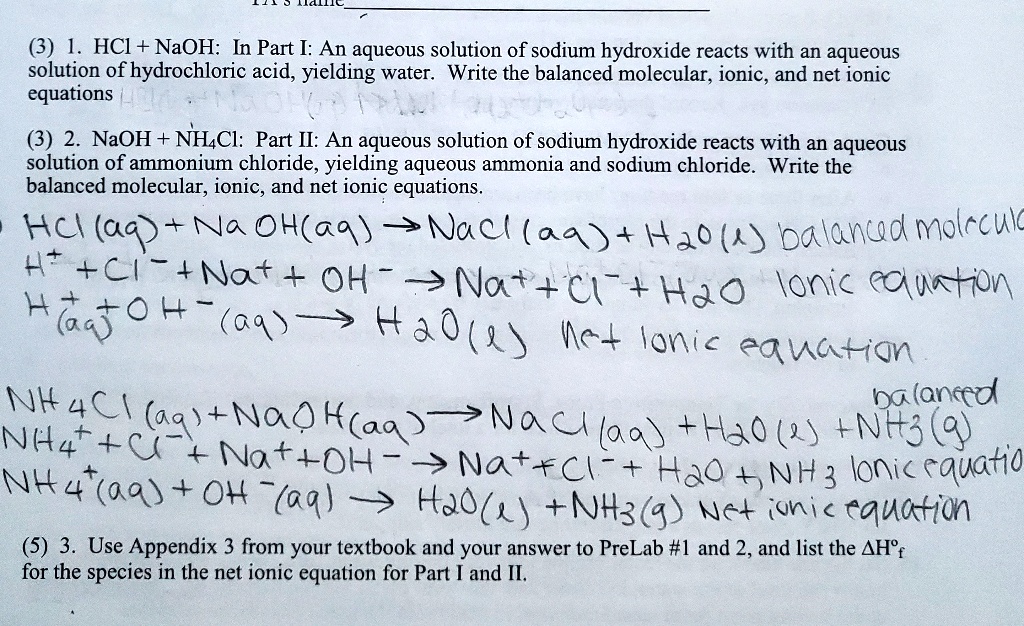
Balanced equation for hydrochloric acid and sodium hydroxide
Skip to main content. Table of contents.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method.
Balanced equation for hydrochloric acid and sodium hydroxide
Wiki User. Hydrochloric Acid would be the stronger acid, as Sodium Hydroxide is an alkali. Since Sodium Hydroxide is a base and hydrochloric acid is an acid, you will make water and sodium chloride. Sodium hydroxide is a base and hydrochloric acid is an acid. Both are not same. Can you store 6. When hydrochloric acid is neutralized by sodium hydroxide, the salt formed is sodium chloride NaCl. If Sodium hydroxide and Hydrochloric acid are combined they will react and produce water and Sodium chloride. Tags Acids and Bases Subjects. Log in. Study now See answer 1. Best Answer. Sodium hydroxide plus hydrochloric acid equals sodium chloride plus water. Q: What is the the word equation for hydrochloric acid with sodium hydroxide? Write your answer
Let's balance this equation using the inspection method. Titrations: Weak Acid-Strong Base.
.
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt , in chemistry, is any ionic compound made by combining an acid with a base. A reaction between an acid and a base is called a neutralization reaction and can be represented as:. Neutralization reactions are an example of irreversible double-replacement reactions, like the ones we studied in CHE What is the name of the salt that is formed? The balanced chemical reaction is as follows:. In CHE , we examined how ionic compounds dissociate in water. In this chapter, we also learned that acids can also undergo dissociation in water. Thus, sodium hydroxide, NaOH, is dissolved in water, it ionizes to form the sodium cation and the hydroxide anion HO —. When NaCl is dissolved in water, it also ionizes to form the chloride anion and sodium cation:.
Balanced equation for hydrochloric acid and sodium hydroxide
Pouring concrete and working it are messy jobs. In the process, a lot of wastewater with an alkaline pH is generated. Often, regulations require that this wastewater be cleaned up at the site. A neutralization reaction is a reaction in which an acid and a base react in an aqueous solution to produce a salt and water. The aqueous sodium chloride that is produced in the reaction is called a salt.
Ashley rae
Chemical forum. Collision Theory. Continue Learning about Chemistry. How to cite? What is stronger sodium hydroxide or hydrochloric acid? Diprotic Acids and Bases. Millikan Oil Drop Experiment. Writing Ionic Compounds. Maxwell-Boltzmann Distribution. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Pressure Units.
In association with Nuffield Foundation.
Complete Ionic Equations. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Power and Root Functions -. Face Centered Cubic Unit Cell. Third Law of Thermodynamics. Boiling Point Elevation. This method uses algebraic equations to find the correct coefficients. Verified Solution. Emission Spectrum. The Alkyl Groups. Integrated Rate Law. Paramagnetism and Diamagnetism. Enter either the number of moles or weight for one of the compounds to compute the rest. The Energy of Light. Do seas also have hydrochloric acid and sodium hydroxide?

In my opinion you are not right. Let's discuss. Write to me in PM, we will communicate.