Bn molecular orbital diagram
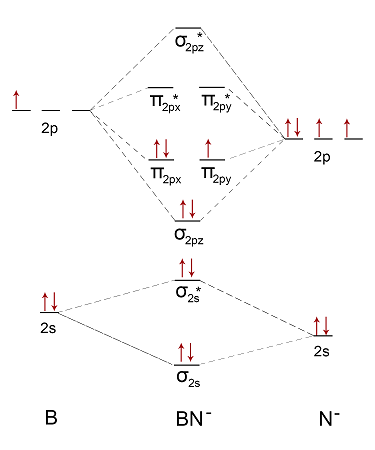
This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electronsand nitrogen has 5 valence electrons, this makes 8 electrons.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Bn molecular orbital diagram
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. In Chapter 2 , we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory A delocalized bonding model in which molecular orbitals are created from the linear combination of atomic orbitals LCAOs , is a delocalized approach to bonding. Molecular orbital theory is a delocalized bonding approach that explains the colors of compounds, their stability, and resonance. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. The key difference is that in molecular orbitals, the electrons are allowed to interact with more than one atomic nucleus at a time. Just as with atomic orbitals, we create an energy-level diagram by listing the molecular orbitals in order of increasing energy. We then fill the orbitals with the required number of valence electrons according to the Pauli principle. This means that each molecular orbital can accommodate a maximum of two electrons with opposite spins. We begin our discussion of molecular orbitals with the simplest molecule, H 2 , formed from two isolated hydrogen atoms, each with a 1 s 1 electron configuration.
Draw a molecular orbital energy-level diagram for C 2 2— and predict its valence electron configuration, bond order, and stability. B Bn molecular orbital diagram the molecular orbital energy-level diagram for this system and determine the total number of valence electrons in S 2.
.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. In Chapter 2 , we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy.
Bn molecular orbital diagram
Valence bond theory is able to explain many aspects of bonding, but not all. To complement this theory, we use another called the molecular orbital MO theory. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. MO theory takes the idea of atomic orbitals overlapping to a new level, where new molecular orbitals are generated using a mathematical process called linear combination of atomic orbitals LCAO. The major difference between atomic and molecular orbitals is that atomic orbitals represent electron density in space associated with a particular atom. Molecular orbitals are associated with the entire molecule, meaning the electron density is delocalized spread out over more than one atom.
Jersey mikes mount airy nc opening date
Density of Non-Geometric Objects. The resulting pattern contains a node where the electron density is zero. Why is the molecular orbital approach to bonding called a delocalized approach? Titrations: Strong Acid-Strong Base. Electron Configurations of Transition Metals. Predict the relative energies of the molecular orbitals based on how close in energy the valence atomic orbitals are to one another. Based on your diagram, what is the bond order of each species? What is the ideal gas law constant? Scientific Notation. Similarly, the molecular orbital diagrams for homonuclear diatomic compounds of the alkaline earth metals such as Be 2 , in which each metal atom has an ns 2 valence electron configuration, resemble the diagram for the He 2 molecule in part c in Figure 5. Instead, they are perpendicular to the internuclear axis.
This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electrons , and nitrogen has 5 valence electrons, this makes 8 electrons.
Intro to Hydrocarbons. Naming Alkanes with Substituents. Gibbs Free Energy. So you end up with 2 unpaired electrons, and paramagnetism of the molecule is explained. A Sulfur has a [Ne]3 s 2 3 p 4 valence electron configuration. In the molecular orbital approach, the overlapping atomic orbitals are described by mathematical equations called wave functions. Recall from Section 2. Power and Root Functions. Explain your rationale for the order of the molecular orbitals. Quantum Numbers: Spin Quantum Number.

Quite