Bond order of n2o
The differences here include the number of oxygens attached to nitrogen. Notice the bonding patterns:. Based on the "N"-"O" bond distances of
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Bond order of n2o
.
Electron Configurations of Transition Metals: Exceptions.
.
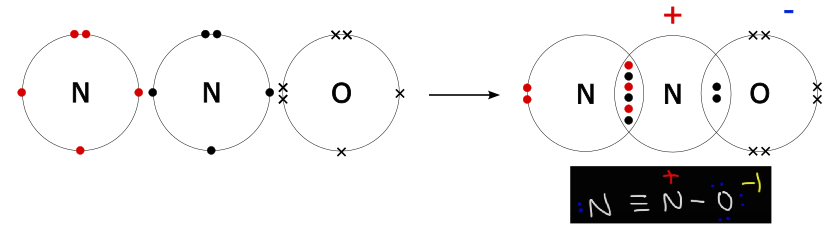
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom. The left nitrogen atom with a triple bond has one lone pair, and the oxygen atom with a single bond has three lone pairs. In the periodic table , nitrogen lies in group 15, and oxygen lies in group Hence, nitrogen has five valence electrons and oxygen has six valence electrons. Learn how to find: Nitrogen valence electrons and Oxygen valence electrons. We have a total of 16 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here, we have a total of 8 electron pairs. And two bonds are already marked.
Bond order of n2o
The Oxygen atom has 3 lone pairs and the outer nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of N2O. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Nitrogen is a group 15 element on the periodic table.
Nike dunk low 2022
Parts per Million ppm. Naming Esters. Thermochemistry 2h 30m. Hydrohalogenation Reactions. Aldehydes and Ketones Reactions. Phase Diagrams. Heat Capacity. Naming Alkanes. Significant Figures. Photoelectric Effect. Molecular Equations.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp 3 hybridized respectively.
Density of Non-Geometric Objects. Hydrogenation Reactions. Heat Capacity. Band of Stability: Overview. The Colligative Properties. Production of Hydrogen. Lattice Energy. Solubility Rules. Boiling Point Elevation. Ester Reactions: Saponification. Naming Alkanes with Substituents. How do chemical bonds affect the properties of a substance? Multiplication and Division Operations. Molecular Geometry.

I apologise, but, in my opinion, you are not right. I can prove it.