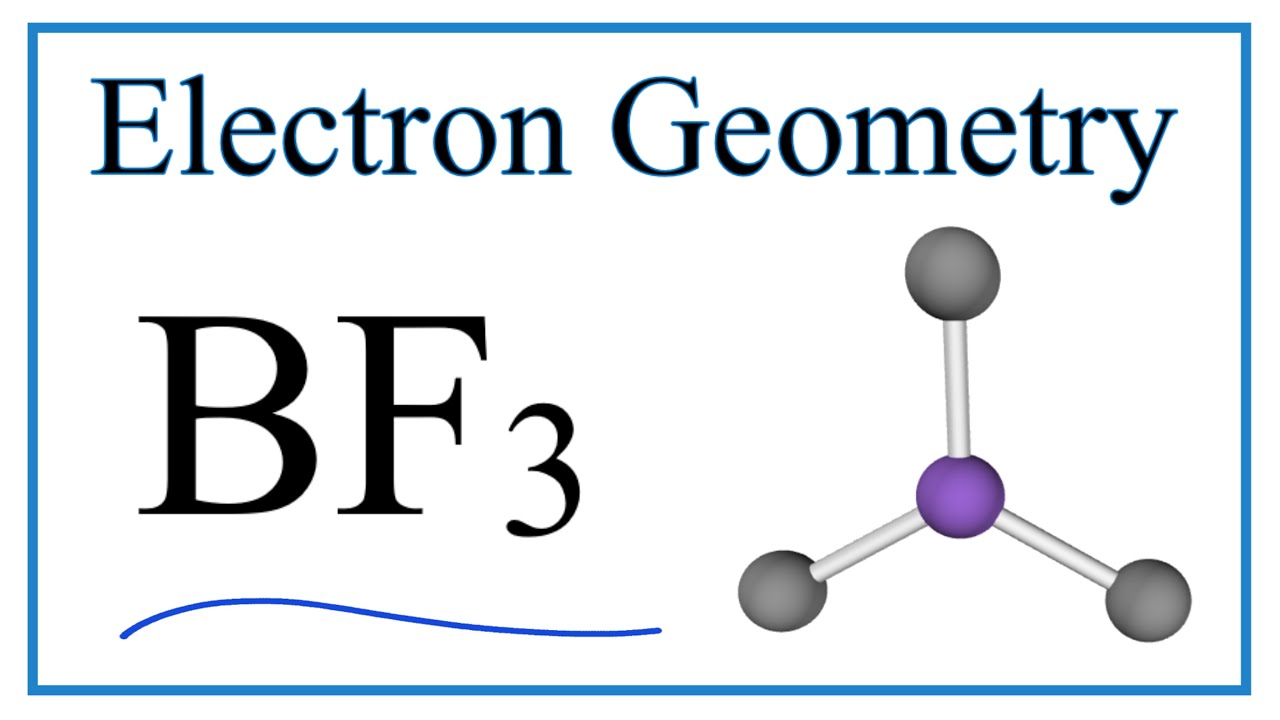
Boron trifluoride shape
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms.
Total: 0. Colourless, heavier-than-air gas with a pungent odour. It forms white fumes in moist air. Boron trifluoride is a colourless , toxic gas with a pungent smell and greater density than air. It forms white smoke during hydrolysis caused by exposure to humid air. It dissolves well in water while forming hydrogen and boric acid.
Boron trifluoride shape
The valence bond theory also predicts a planar triangle with hybridisation of one s and two p orbitals used for bonding. However, the B atom only has six electrons in its outer shell and this is termed electron deficient. The empty 2p z atomic orbital on B which is not involved in hybridisation is perpendicular to the triangle containing the sp 2 hybrid orbitals. This p z orbital may accept an electron pair from a full p z orbital on any one of the three fluorine atoms. If one localized double bond existed, then there would be one short bond and two longer ones. However, all measurements show that the three bond lengths are identical. The old valence bond explanation of this was resonance between three structures with the double bond in different positions. The modern explanation is that the double bond is delocalised. The order is the reverse of what would be normally expected on the basis of electronegativity of halogen and also on the basis of steric grounds. Home What is the boron trifluoride formula? What is the boron trifluoride formula? Related Chapters How to calculate equivalent weight?
How Haloform reaction proceed?
In this article, you will read about BF3 molecular geometry. The inorganic compound is boron trifluoride with formula BF 3. BF 3 is colourless, poisonous gas that has no colour. In damp air, it releases white vapours and is soluble if it is in the form of a colourless liquid i. This plane seems like all peripheral atoms exist in one place. For determining the lewis structure, you need to calculate the total number of valence electrons for the BF 3 molecule.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table. Data evaluated as indicated in comments: L - Sharon G. Lias, John E. Bartmess, Joel F.
Boron trifluoride shape
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest inert gas neighbor, neon. Boron and fluorine will combine to form three B-F single covalent bonds. Boron uses all its three valence electrons to bond with the three fluorine atoms, leaving no lone pairs of electrons.
Lmanburg flag
If you follow all the steps, you can easily draw the structure of BF 3 Hybridization. Polarity refers to the separation of electric charge in an electric dipole or multipole moment in a molecule or its corresponding groups. Related Chapters How to calculate equivalent weight? Oxalic-Acid vs KMnO4. The old valence bond explanation of this was resonance between three structures with the double bond in different positions. Actinides Guide. Universal Indicator. Acids definition. Fluorine is located in Group 17 and has seven valence electrons. Log in. LiAlH4 Reaction and Mechanism. Access more than. Aluminium silicate zeolites are microporous three-dimensional crystalline solids.
Boron Trifluoride BF3 is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule.
Atomic weight of elements. Exception 1 : If the octet has an odd number of valence electrons like 3,5,7, etc. Dash lines represent single covalent bonds. Heisenberg's Uncertainty Principle. In damp air, it releases white vapours and is soluble if it is in the form of a colourless liquid i. Boron is located in Group 13 of the periodic table. How to balance a chemical equation. Boron trifluoride is used in the production of other boron compounds ; it is also used as a catalyst and in fumigation. As a result, you can say the BF 3 molecule is nonpolar. Conclusion In this article, we learned how to sketch BF 3 molecular geometry and found the process of finding the lone pairs of electrons in the central boron atom, along with BF 3 hybridization and BF 3 molecular notation. Access free live classes and tests on the app. What is the structure of diboranes? What is the boron trifluoride formula? It also includes three fluorine atoms; the BF 3 molecule exhibits a trigonal planar geometric shape. In this article, we learned how to sketch BF 3 molecular geometry and found the process of finding the lone pairs of electrons in the central boron atom, along with BF 3 hybridization and BF 3 molecular notation.

I can believe to you :)
Should you tell you on a false way.