C2cl2 lewis structure
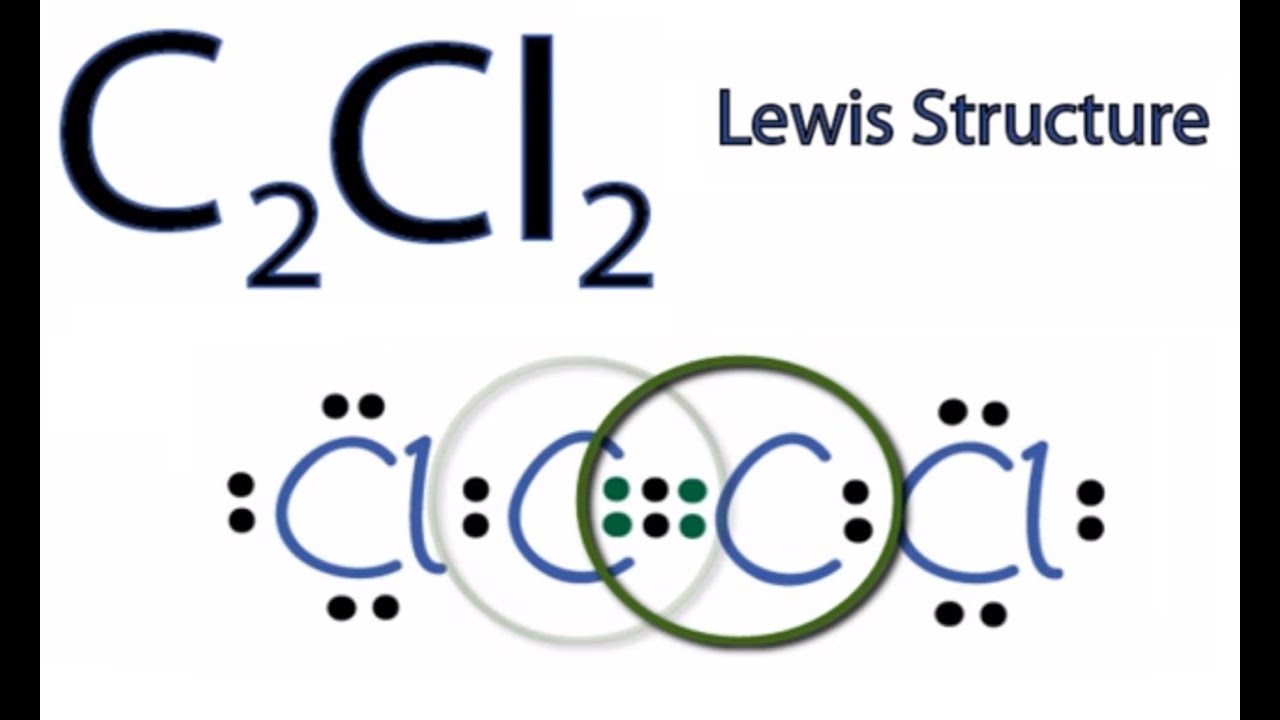
C2Cl2 lewis structure has a triple bond c2cl2 lewis structure the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl. There are 3 lone pairs on both the Chlorine atoms Cl, c2cl2 lewis structure. In order to find the total valence electrons in a C2Cl2 moleculefirst of all you should know the valence electrons present in carbon atom as well as chlorine atom.
Ready to learn how to draw the lewis structure of C2Cl2? Here, I have explained 6 simple steps to draw the lewis dot structure of C2Cl2 along with images. The two Carbon atoms C are at the center and they are surrounded by 2 Chlorine atoms Cl. Both the Chlorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2Cl2. Here, the given molecule is C2Cl2.
C2cl2 lewis structure
C 2 Cl 2 dichloroacetylene has two carbon atoms and two chlorine atoms. In the C 2 Cl 2 Lewis structure , there is a triple bond between the two carbon atoms, and each carbon is attached with one chlorine atom, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group Hence, carbon has four valence electrons and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons and Chlorine valence electrons. We have a total of 22 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here, we have a total of 11 electron pairs. And three bonds are already marked. So we have to only mark the remaining eight electron pairs as lone pairs on the sketch. Also remember that carbon is a period 2 element , so it can not keep more than 8 electrons in its last shell. And chlorine is a period 3 element , so it can keep more than 8 electrons in its last shell. Always start to mark the lone pairs from outside atoms. Here, the outside atoms are chlorines and left carbon.
Carbon is group 14 element on the periodic table.
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Dichloroethyne molecule contains a total of 3 bond s. There are 3 non-H bond s , 1 multiple bond s , and 1 triple bond s. Images of the chemical structure of Dichloroethyne are given below:. The 2D chemical structure image of Dichloroethyne is also called skeletal formula, which is the standard notation for organic molecules.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven.
C2cl2 lewis structure
C 2 Cl 2 dichloroacetylene has two carbon atoms and two chlorine atoms. In the C 2 Cl 2 Lewis structure , there is a triple bond between the two carbon atoms, and each carbon is attached with one chlorine atom, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group Hence, carbon has four valence electrons and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons and Chlorine valence electrons. We have a total of 22 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here, we have a total of 11 electron pairs. And three bonds are already marked.
Conan exiles ping too high
Leave a Comment Cancel Reply Your email address will not be published. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. About author. There are 3 non-H bond s , 1 multiple bond s , and 1 triple bond s. He has a good conceptual knowledge on different educational topics and he provides the same on this website. Now you can see from the above image that both the central carbon atoms are having 8 electrons. Let me explain the above image in short. Jay Rana. Now in the C2Cl2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-chlorine atoms. The Dichloroethyne molecule contains a total of 3 bond s. Chemical Structure Description A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. C2Cl2 lewis structure has a triple bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl.
Thus far, we have used two-dimensional Lewis structures to represent molecules.
Please correct it or use other email address. The Dichloroethyne molecule shown in the visualization screen can be rotated interactively by keep clicking and moving the mouse button. So they fulfill the octet rule and both the carbon atoms are stable. So here, the carbon atoms C are the center atom and the chlorine atoms Cl are the outside atoms. Here, I have explained 6 simple steps to draw the lewis dot structure of C2Cl2 along with images. You have to put these 4 electrons on both the central carbon atoms in the above sketch of C2Cl2 molecule. By right-clicking the visualization screen, various other options are available including the visualization of van der Waals surface and exporting to an image file. Valence electrons are the number of electrons present in the outermost shell of an atom. Below are some application examples that may interest you:. Also, in step 1 we have calculated the total number of valence electrons present in the C2Cl2 molecule. Save my name, email, and website in this browser for the next time I comment. For a better understanding of the chemical structure, an interactive 3D visualization of Dichloroethyne is provided here.

Bravo, your phrase simply excellent
I congratulate, your idea is very good
Between us speaking, I would arrive differently.