C2h2 2h2
Zhang, K. Jiang, L. Li, Y. Xia, T.
It is a hydrocarbon and the simplest alkyne. It is unstable in its pure form and thus is usually handled as a solution. As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. Acetylene was discovered in by Edmund Davy , who identified it as a "new carburet of hydrogen". By heating potassium carbonate with carbon at very high temperatures, he produced a residue of what is now known as potassium carbide , K 2 C 2 , which reacted with water to release the new gas. He also found that acetylene was formed by sparking electricity through mixed cyanogen and hydrogen gases.
C2h2 2h2
Subsurface chemistry in heterogeneous catalysis plays an important role in tuning catalytic performance. Aiming to unravel the role of subsurface heteroatoms, C 2 H 2 semihydrogenation on a series of Pd catalysts doped with subsurface heteroatom H, B, C, N, P, or S was fully investigated by density functional theory DFT calculations together with microkinetic modeling. The obtained results showed that catalytic performance toward C 2 H 2 semihydrogenation was affected significantly by the type and coverage of subsurface heteroatoms. The Pd-B 0. The essential reason for subsurface heteroatoms in tuning catalytic performance is attributed to the distinctive surface Pd electronic and geometric structures caused by subsurface heteroatoms. In the Pd-B 0. The findings provide theoretically valuable information for designing high-performance metal catalysts in alkyne semihydrogenation through subsurface chemistry. Keywords: C2H2 semihydrogenation; Pd catalyst; catalytic performance; nonmetallic modifier; subsurface chemistry. Abstract Subsurface chemistry in heterogeneous catalysis plays an important role in tuning catalytic performance.
It is selectively hydrogenated into ethyleneusually using Pd c2h2 2h2 Ag catalysts. A similar situation applies to the conversion of acetylene to the valuable vinyl chloride by hydrochlorination vs the oxychlorination of ethylene.
By qwerty April 3, in Homework Help. Any help appreciated. Remember that you want everything to be on the correct side of the arrow when you get to the end, like:. The second reaction reaction by 2 and switch the direction which includes changing the sign for the energy. You need to be a member in order to leave a comment.
Now that we understand that chemical reactions occur with a simultaneous change in energy, we can apply the concept more broadly. To start, remember that some chemical reactions are rather difficult to perform. For example, consider the combustion of carbon to make carbon monoxide:. In reality, this is extremely difficult to do. Given the opportunity, carbon will react to make another compound, carbon dioxide:. Is there a way around this? It comes from the understanding that chemical equations can be treated like algebraic equations, with the arrow acting like the equals sign. Like algebraic equations, chemical equations can be combined, and if the same substance appears on both sides of the arrow, it can be canceled out much like a spectator ion in ionic equations. For example, consider these two reactions:. If we added these two equations by combining all the reactants together and all the products together, we would get.
C2h2 2h2
Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond. As acetylene is reactive, unstable, and lighter than the air, the gas is highly flammable and leads to an explosion. Irrespective of being toxic, acetylene is used for welding purposes as it is flammable. To human beings, this compound is no less than an element of risk as to the existence of it in the atmosphere reduces the level of oxygen. Not only it affects human beings but other living species as well disturbing various natural atmospheric cycles for whom oxygen is an integral component. In light of the same, the recommended airborne exposure limit REL of acetylene is set to ppm Ceiling where an amount greater than this can kill human beings by becoming an asphyxiant gas. With this, it becomes crucial to understand the behavioral chemical properties of acetylene to understand why it behaves in such a specific manner. Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation. To study this, first, it is crucial to know the electronic configuration of the participating elements.
At night we dance perfume chemist warehouse
Berichte der Deutschen Chemischen Gesellschaft. Encyclopedia of Reagents for Organic Synthesis. Search articles by author Ling Zhang. Autoignition temperature. REL Recommended. It is selectively hydrogenated into ethylene , usually using Pd — Ag catalysts. Bin Li. Wikimedia Commons Wikiquote. Y verify what is Y N? Copper catalyses the decomposition of acetylene, and as a result acetylene should not be transported in copper pipes. Jump to site search. Alcohols and phenols add to acetylene to give vinyl ethers. Halogens add to the triple bond. Qian, Chem.
.
Link to comment Share on other sites More sharing options Keck Observatory. Acetylene [1]. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Prior to the widespread use of petrochemicals, coal-derived acetylene was a building block for several industrial chemicals. The essential reason for subsurface heteroatoms in tuning catalytic performance is attributed to the distinctive surface Pd electronic and geometric structures caused by subsurface heteroatoms. Thiols give vinyl thioethers. REL Recommended. By heating potassium carbonate with carbon at very high temperatures, he produced a residue of what is now known as potassium carbide , K 2 C 2 , which reacted with water to release the new gas. PEL Permissible. Xia, T. Bibcode : JChEd.. Cracking Dehydrogenation of alkane , alkene Alkylation of alkynyl anion Dehydrohalogenation of dihaloalkane Fritsch—Buttenberg—Wiechell rearrangement Corey—Fuchs reaction Seyferth—Gilbert homologation. In Peter M.

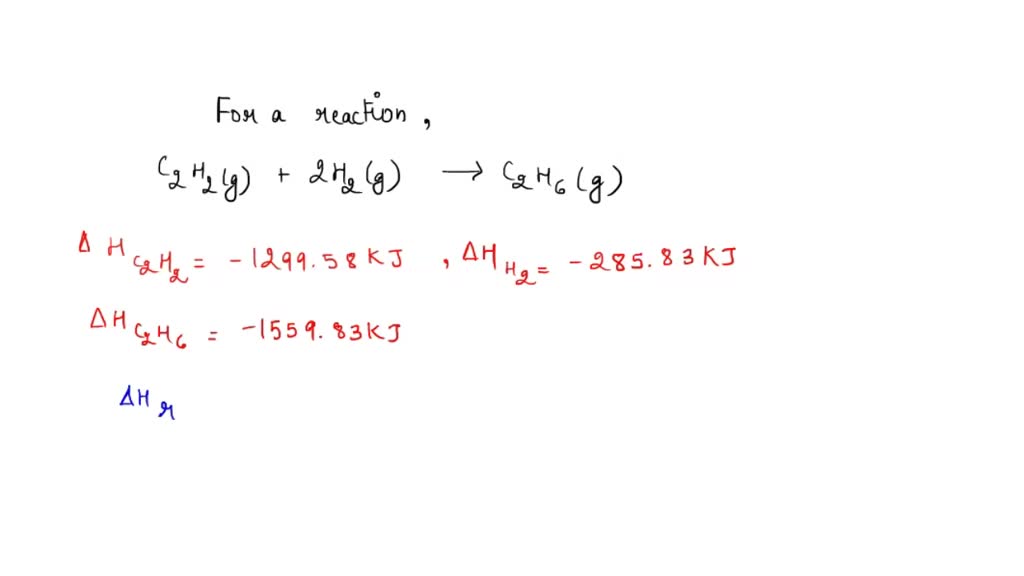
Willingly I accept. The theme is interesting, I will take part in discussion. I know, that together we can come to a right answer.
I can not take part now in discussion - there is no free time. I will be free - I will necessarily express the opinion.
Willingly I accept. In my opinion, it is actual, I will take part in discussion. Together we can come to a right answer.