Calcium hydroxide molar mass
With an accout for my.
Q: How many moles of aluminum are needed to make 6 moles of H2? Give just the number with the correct…. Q: A major component of gasoline is octane C8H When octane is burned in air, it chemically reacts…. Q: What is the mass, in grams, of 1.
Calcium hydroxide molar mass
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0. With a solubility product K sp of 5. At ambient temperature, calcium hydroxide portlandite dissolves in water to produce an alkaline solution with a pH of about Calcium hydroxide solutions can cause chemical burns. At high pH values due to a common-ion effect with the hydroxide anion , its solubility drastically decreases. This behavior is relevant to cement pastes. Aqueous solutions of calcium hydroxide are called limewater and are medium-strength bases , which react with acids and can attack some metals such as aluminium [ citation needed ] amphoteric hydroxide dissolving at high pH , while protecting other metals, such as iron and steel , from corrosion by passivation of their surface.
The properties are an average of those of chemical A and chemical B. The molar mass of calcium hydroxide, Ca OH 2, is O Matter and Measurement In the universe, there is matter, energy, and void space.
Questions Courses. The molar mass of calcium hydroxide, Ca OH 2, is O May 25 AM. Subhash P answered on May 27, Do you need an answer to a question different from the above?
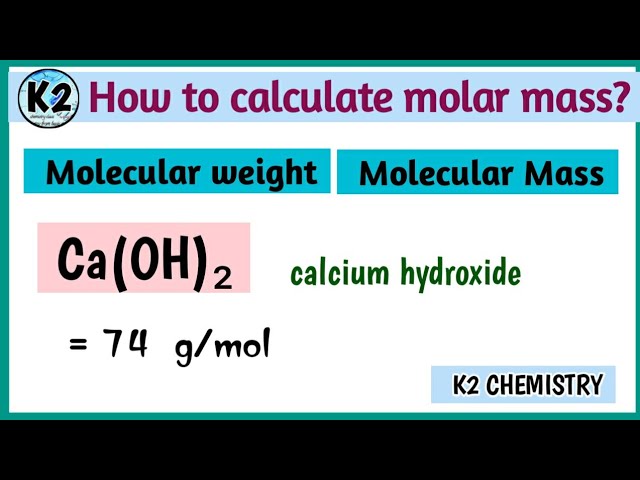
Molar mass of Calcium hydroxide [Ca OH 2] is Let me show you the calculation to get the molar mass of Ca OH 2 or Calcium hydroxide. If you have a periodic table with you, then you can easily calculate the molar mass of Ca OH 2 or Calcium hydroxide. Because the molar mass of any molecule or compound can be calculated by simply adding the molar masses of individual atoms. The molar mass of Calcium is The molar mass of Oxygen is The molar mass of Hydrogen is 1. Now, to calculate the molar mass of Ca OH 2, you just have to add the molar mass of all the individual atoms that are present in Ca OH 2. Hence the Molar mass of Ca OH 2 is
Calcium hydroxide molar mass
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. The structure of a Ca OH 2 molecule is illustrated below. Unprotected exposure to this compound can prove dangerous to humans, leading to irritation of the skin and chemical burns. Exposure to concentrated Ca OH 2 can lead to lung damage and even blindness.
Dianne feinstein dead meme
When elemental carbon is burned in the open atmosphere, with plenty of oxygen gas present, the product is carbon dioxide. Problem 14QAP: For each of the following balanced chemical equations, calculate how many moles and how many grams Problem 54QAP: If steel wool iron is heated until it glows and is placed in a bottle containing pure oxygen, the Molecular formula. In the universe, there is matter, energy, and void space. Zr OH 4. Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. Review Please. In other words, the mole is the amount of substance equal in mass to the combined mass in atomic mass units of the atoms of molecules of the substance multiplied by the Avogadro constant or Avogadro number. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Q: The chemical formula for t-butanol is: CH; , Calculate the molar mass of t-butanol. A: Empirical formula is the representation of chemical formula in which gives the simplest whole number…. Round your answer to 2 decimal places.
Calcium dihydroxide.
A: The mass of one mole of a substance is known as molar mass. Problem 92AP: Before going to lab, a student read in his lab manual that the percent yield for a difficult He finds that Problem 54QAP: If steel wool iron is heated until it glows and is placed in a bottle containing pure oxygen, the Retrieved 17 July Lattice constant. A: To determine average percentage of acetic acid in Vinegar. Unit converters. The chemical formula for calcium hydroxide is: Ca OH 2 Calculate the molar mass of calcium hydroxide. Problem 35QAP: Elemental phosphorus bums in oxygen with an intensely hot flame, producing a brilliant light and The structure is identical to that of Mg OH 2 brucite structure ; i. The molecular weight of acetic acid is

I am sorry, that has interfered... At me a similar situation. Write here or in PM.
I to you will remember it! I will pay off with you!
I apologise, but, in my opinion, you commit an error. Let's discuss it. Write to me in PM.