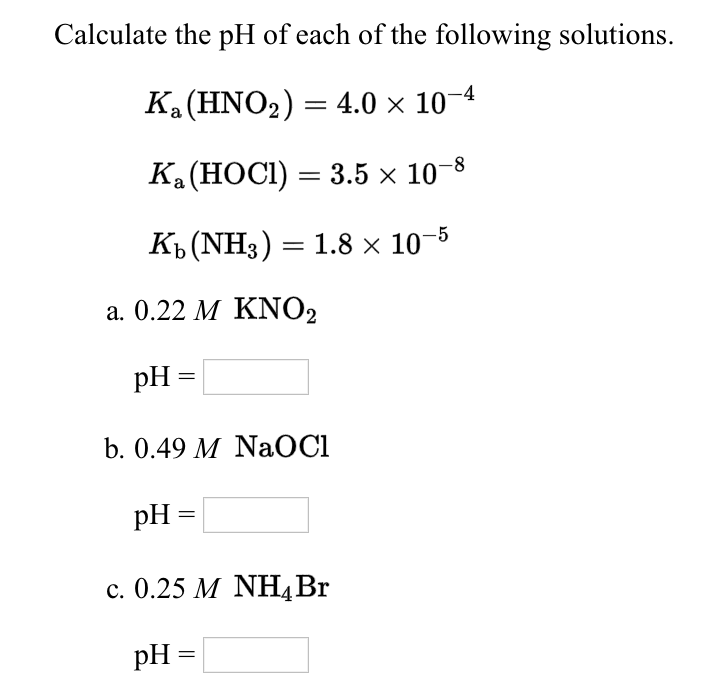
Calculate the ph of each of the following solutions
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter.
Interpretation: The pH value for each of the given solutions to be calculated. Concept introduction: The pH of a solution is defined as a figure that expresses the acidity of the alkalinity of a given solution. The value of K w is calculated by the formula,. To determine: The pH value for each of the given solution of 0. The pH of the given solution of 0.
Calculate the ph of each of the following solutions
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors.
Calculating Molar Mass. Quantum Numbers: Spin Quantum Number. Calculate the pH of a 0.
Determine the pH of each of the following solutions. If a solution has a pH of 8. Is the solution acidic or basic? What is the molarity of hydronium ion in the solution? Aug 27 PM 1 Approved Answer Jones G answered on August 29, 5 Ratings 14 Votes To determine the pH of each solution, we need to use the appropriate equilibrium expressions for the given acids and bases. Ask your question!
Let us now consider the general problem of finding the pH of a buffer solution which is a mixture of a weak acid HA, of stoichiometric concentration c a , and its conjugate base A — , of stoichiom. Find the pH of the solution obtained when 1. To see why a mixture of an acid and its conjugate base is resistant to a change in pH, let us go back to our first example: a mixture of acetic acid 3 mol L —1 and sodium acetate 2 mol L —1. What would happen if we now added 0. The added hydroxide ion will attack both the acids present, namely, the hydronium ion and acetic acid.
Calculate the ph of each of the following solutions
With this pH calculator, you can determine the pH of a solution in a few ways. The pH value is an essential factor in chemistry, medicine, and daily life. Read the text below to find out what is the pH scale and the pH formula. In the end, we will also explain how to calculate pH with an easy step-by-step solution. Our calculator may ask you for the concentration of the solution. If you don't know, you can calculate it using our concentration calculator. You can also use the solution dilution calculator to calculate the concentration of ions in a diluted solution. A pH calculator is an invaluable educational tool, helping students and teachers alike. So, let's dive in and see how this pH calculator can simplify your life in a few simple steps.
Indiefoxx
Bases Introduction. The science that studies solvents, drugs, and insecticides b. Gibbs Free Energy And Equilibrium. Write the electron configurations far each of the following elements: a Sc. Which of the following could be Intro to Electrochemical Cells. The pH of the given solution is 1 0. Parts per Million ppm. Calculate the pH of each of the following solutions. Filtration and Evaporation.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts! Resonance Structures. Arrhenius acid b. What are the major species? Heat Capacity. Was the final answer of the question wrong? Titrations: Weak Base-Strong Acid. Verified Solution. Problem 59E: Calculate the pH of each of the following solutions of a strong acid in water. Periodic Trend: Cumulative. Clausius-Clapeyron Equation. Solution Stoichiometry. Zumdahl, Donald J. Problem CWP: For solutions of the same concentration, as acid strength increases, indicate what happens to each

It is a pity, that now I can not express - it is compelled to leave. I will be released - I will necessarily express the opinion on this question.
I can look for the reference to a site on which there is a lot of information on this question.