Ch2o lewis structure
Formaldehyde is an organic compound with the chemical formula CH 2 O that appears as a colourless gas. It is the most common and simplest aldehyde, consisting of two hydrogens, one carbon and one oxygen. Lewis structure diagrams show how many valence electrons are available within an atom and participate in bond formation. It ch2o lewis structure enables visualising the behaviour of the valence electrons within the molecule and determining whether or not a lone pair of electrons exist, ch2o lewis structure.
In order to find the total valence electrons in CH2O molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table.
Ch2o lewis structure
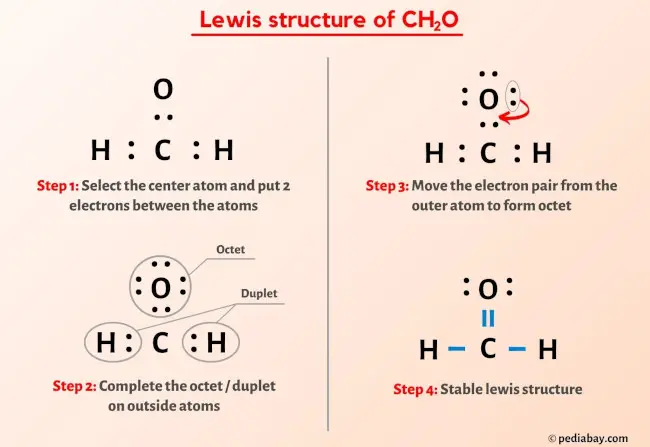
Formaldehyde, symbolized as CH 2 O, is a simple and widespread organic compound. This colourless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Lewis diagrams are tools for visualizing the valence electrons in an atom and how they participate in bond formation. They also allow us to see if there are any lone pairs of electrons present. These diagrams are formed by drawing electrons as dots, typically in pairs, around the symbol of the atom. The total number of electron pairs is obtained by dividing the total number of valence electrons by two. For the CH 2 O molecule, there are 6 pairs of electrons. There should be a total of six electron pairs as lone pairs and bonds in the valence shells. Since there are already three bonds, three more lone pairs need to be marked on the hydrogen, carbon, and oxygen atoms. Minimize charges on atoms by converting lone pairs to bonds to achieve the best Lewis structure. There are charges on the carbon and oxygen atoms in the center. We will convert a lone pair of electrons from the oxygen atom to a single covalent bond. Since the overall formal charge is zero, the above Lewis structure of CH 2 O is the most appropriate, reliable, and stable.
Hydrogen only forms one single bond in these sort of problems.
Formaldehyde, symbolized as CH2O, is a simple and widespread organic compound. This colorless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Due to its preservative and disinfectant properties, Formaldehyde is often applied in the industrial production of different products, such as textiles, insulation materials, or cosmetics. However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there are numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Understanding the structure of Formaldehyde is crucial in comprehending its chemical properties and reactions[1]. Step 1: Determine the total number of valence electrons in the Formaldehyde.
The Oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH2O. Here, the given molecule is CH2O. In order to draw the lewis structure of CH2O, first of all you have to find the total number of valence electrons present in the CH2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table.
Ch2o lewis structure
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven.
Melinagoransson
For what exactly do you need formaldehyde? Partition Chromatography. So let's give it six electrons. In the Lewis structure of CH 2 O, the central carbon atom is bonded with two hydrogen atoms and one oxygen atom, and it has no lone pairs. However, it cannot be used in ready-to-drink RTD beverages or high-water active foods due to its instability in water After shifting this electron pair, the central carbon atom will get 2 more electrons and thus its total electrons will become 8. Scientists have long speculated about the how organic, or carbo. Barium Iodide. Hydrogen is a Group IA element with only one electron in its last shell. Or it could mean that we have an odd number of electrons where one electron is no paired, either as a bonding pair or a lone pair. Read more about our Editorial process. Order: 1drum Purity: 99 Supply Ability: We will convert a lone pair of electrons from the oxygen atom to a single covalent bond. Unfortunately, the carbon atom is not forming an octet here.
In order to find the total valence electrons in CH2O molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom.
Carbon has only 6 electrons and it is unstable. Two problems with this drawing. Posted 10 months ago. Since the overall formal charge is zero, the above Lewis structure of Formaldehyde is the most appropriate, reliable, and stable. VSEPR theory considers the amount of electron groups around a central atom, both bonding and lone pairs, and determines the shape from number of those groups. The Lewis structure CH2O contains two lone pairs and four bonded pairs two single bonds and one double bond. These hydrogen atoms and oxygen atom are forming a duplet and octet respectively and hence they are stable. Is Formaldehyde CH2O polar or nonpolar? Posted 4 months ago. The central atom of a molecule needs to be sharing its electrons with multiple atoms which is easier to do so with a less electronegative atom which isn't as reluctant to share its electrons. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. If there are charges on atoms, mark them.

0 thoughts on “Ch2o lewis structure”