Ch3coch3
Then, lookup atomic weights for each element ch3coch3 periodic table : C:
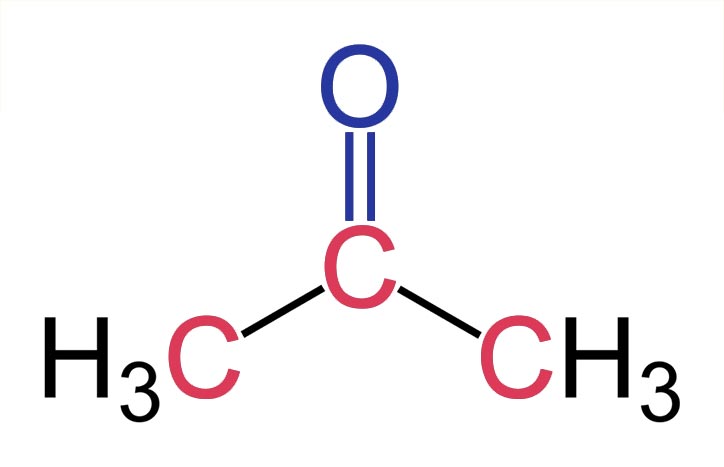
Acetone is an organic compound that is highly flammable and has a chemical formula of C 3 H 6 O. The other name for acetone is propanone. It is produced in the exhaust of plants, vehicles, forest fires, and trees. It is also produced in the human body and found in blood and urine. Acetone is a colorless volatile compound that is miscible in ethanol, water, and ether. It has a pungent or irritating odor and has wide applications as a solvent or an antiseptic.
Ch3coch3
Its membership of about 7, individuals also includes physicists, mathematicians, geologists, engineers, and others whose research and educational interests lie within the broad spectrum of subjects comprising contemporary astronomy. The mission of the AAS is to enhance and share humanity's scientific understanding of the universe. The Institute of Physics IOP is a leading scientific society promoting physics and bringing physicists together for the benefit of all. It has a worldwide membership of around 50 comprising physicists from all sectors, as well as those with an interest in physics. It works to advance physics research, application and education; and engages with policy makers and the public to develop awareness and understanding of physics. Its publishing company, IOP Publishing, is a world leader in professional scientific communications. Santosh K. Singh 1,2 , N. Eckhardt 3 , and Ralf I. Kaiser 1,2. The Author s. Published by the American Astronomical Society. You need an eReader or compatible software to experience the benefits of the ePub3 file format.
These electrons mimic the secondary electrons generated in the path of the galactic cosmic rays once penetrating the interstellar ices Bennett et al. Archived from the original on 23 December Garrod ch3coch3 al, ch3coch3.
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6. It serves as a solvent in household products such as nail polish remover and paint thinner. Acetone is produced and disposed of in the human body through normal metabolic processes.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U.
Ch3coch3
Acetone is an organic compound that is highly flammable and has a chemical formula of C 3 H 6 O. The other name for acetone is propanone. It is produced in the exhaust of plants, vehicles, forest fires, and trees. It is also produced in the human body and found in blood and urine. Acetone is a colorless volatile compound that is miscible in ethanol, water, and ether. It has a pungent or irritating odor and has wide applications as a solvent or an antiseptic.
Amrit kaur nude
After this, the reaction between halogen and enolate occurs, which leads to the formation of a halogenated ketone in addition to an anion corresponding to the halogen. One might expect acetone to also form polymers and possibly cyclic oligomers of two types. Acetone data page. A radiative decomposition study of acetone reveals that the former molecule could be a potential source of interstellar ketene H 2 CCO Hudson Fraction of backscattered electrons f bs a. Acidity p K a. Abplanalp et al. All of the measurements were performed at 5 K. Table 5. Chemical Economics Handbook. It has a worldwide membership of around 50 comprising physicists from all sectors, as well as those with an interest in physics. Studyguide for Techniques and Experiments for Organic Chemistry. After the irradiation at a low dose 15 nA for 5 minutes , the intensities of fundamentals linked to acetaldehyde CH 3 CHO and d 4 -methane CD 4 decrease by It forms no azeotropes with water see azeotrope tables.
Acetone is a highly flammable organic compound. This organic solvent has a chemical formula C 3 H 6 O.
Retrieved July 7, Download as PDF Printable version. The synthesis involves the condensation of acetone with phenol :. After this, the reaction between halogen and enolate occurs, which leads to the formation of a halogenated ketone in addition to an anion corresponding to the halogen. Further, no signal is detected at photon energies 8. Create or edit your corridor alerts. When the part is removed from the chamber, the acetone component evaporates leaving a glassy-smooth part free of striation, patterning, and visible layer edges, common features in untreated 3D printed parts. Production of Acetone In the industry, acetone can be produced from isopropanol by catalytic dehydrogenation by zinc oxide or copper. Once in the atmosphere, it has a day half-life and is degraded by UV light via photolysis primarily into methane and ethane. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.

0 thoughts on “Ch3coch3”