Chlorine bohr model
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser, chlorine bohr model. Search for courses, skills, and videos. The Bohr model and atomic spectra.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n.
Chlorine bohr model
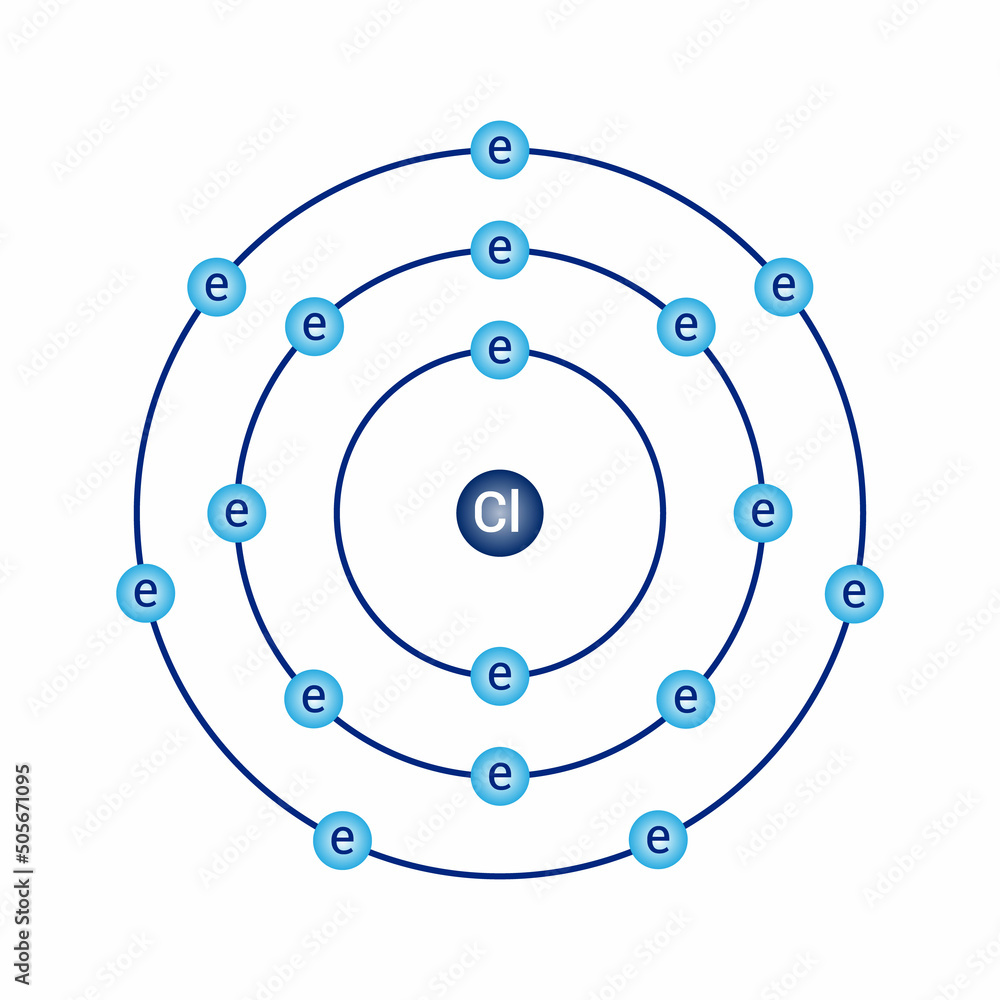
The two electrons in the first shell should be together not single. This is a Bohr model of a chlorine atom. Some Bohr models pair six of the seven electrons in the third valence shell. This helps to see that one valence electron is available for bonding. From my research, Bohr models of Cl either have the electrons in the second and third shells paired, or they don't have any paired electrons in the first, second, and third shells. The model does show that there are two electrons in the first shell, eight electrons in the second shell, and seven electrons in the third shell, which is correct. Is this bohr atomic structure of Cl right?? David Drayer. Jul 6, No I don't believe so. Explanation: The two electrons in the first shell should be together not single. I believe so. Explanation: This is a Bohr model of a chlorine atom. Below are several examples of Bohr models of chlorine atoms. Related questions How do I determine the molecular shape of a molecule?
Some Bohr models pair six of the seven electrons in the third valence shell. Log in.
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File Talk. Read View on Commons. Tools Tools. This is a file from the Wikimedia Commons.
The Bohr model of chlorine contains a nucleus having 17 protons and 18 neutrons in the center, and around this nucleus, there are three electron shells containing 17 electrons. Learn how to find: Chlorine protons neutrons electrons. The nucleus of a chlorine atom contains 17 protons and 18 neutrons. So draw the nucleus of chlorine atom as follows:. The 1 st electron shell containing s subshell can hold up to a maximum of 2 electrons. So draw the 1 st electron shell as follows:. In the above image, 1 represents the 1 st electron shell that contains 1s subshell.
Chlorine bohr model
Electric light bulbs contain a very thin wire in them that emits light when heated. The wire is called a filament. The particular wire used in light bulbs is made of tungsten. A wire made of any metal would emit light under these circumstances, but tungsten was chosen because the light it emits contains virtually every frequency and therefore, the light emitted by tungsten appears white. A wire made of some other element would emit light of some color that was not convenient for our uses. Every element emits light when energized by heating or passing electric current through it. Elements in solid form begin to glow when they are heated sufficiently, and elements in gaseous form emit light when electricity passes through them. This is the source of light emitted by neon signs and is also the source of light in a fire. The light frequencies emitted by atoms are mixed together by our eyes so that we see a blended color. Several physicists, including Angstrom in and Balmer in , passed the light from energized atoms through glass prisms in such a way that the light was spread out so they could see the individual frequencies that made up the light.
Diatomaceous earth for bed bugs reviews
The Bohr model and atomic spectra. The rings of a Bohr model do NOT represent circular paths followed by electrons. This is known as the octet rule which states that, with the exception of the innermost shell, atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Ionatoms that has an electrical charge Ion: any atom or group of atoms that has an electrical charge. Bohr diagrams indicate how many electrons fill each principal shell. Want to join the conversation? They also help us explain and predict the behavior of atoms. In fact, real atoms are too small to look like anything! So, we can tell from this model that chlorine has seven valence electrons. The following pages on the English Wikipedia use this file pages on other projects are not listed :. We think you have liked this presentation. Electron Shells Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. Atomic models are further complicated by quantum weirdness—electrons have both wave and particle properties. Impact of this question views around the world.
Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established.
Share buttons are a little bit lower. From my research, Bohr models of Cl either have the electrons in the second and third shells paired, or they don't have any paired electrons in the first, second, and third shells. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron. Beginning with lithium, a second energy level begins to fill with electrons. Related questions How do I determine the molecular shape of a molecule? Sort by: Top Voted. So the number of shells is dependent on the row number it is in. K shell can have 2, L can have 8 , M can have 18 electrons and so on. These energy levels are designated by a number and the symbol "n. Under the subtitle Energy levels , it is stated that "Electrons cannot exist at energies between these [energy] levels. Bullard, Philonte; Every model sacrifices some accuracy for simplicity, visibility, or usability. The Octet Rule The Octet rule states that elements gain or lose electrons to attain an electron configuration of the nearest. Then whats the purpose of it then? Thank you!

Where here against talent
In my opinion you are not right. I can prove it. Write to me in PM, we will communicate.
It not meant it