Cl2 lewis
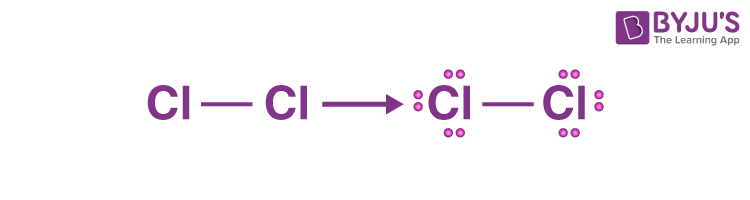
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine moleculecl2 lewis, first of all you should know the valence electrons present in a single chlorine atom. Valence electrons are the electrons cl2 lewis are present in the outermost orbit of any atom.
Chlorine gas, a member of the halogen group, exists in the form of a diatomic molecule with the chemical formula Cl 2. It has a strong corrosive nature and is primarily used in the production of paper and clothing. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell.
Cl2 lewis
Ready to learn how to draw the lewis structure of Cl2? Here, I have explained 6 simple steps to draw the lewis dot structure of Cl2 along with images. Lewis structure of Cl2 Chlorine contains one single bond between both the Chlorine Cl atoms. And both the Chlorine atoms have three lone pairs on it. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of Cl2. Here, the given molecule is Cl2 Chlorine. In order to draw the lewis structure of Cl2, first of all you have to find the total number of valence electrons present in the Cl2 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Chlorine is a group 17 element on the periodic table. But here in the Cl2 molecule, both the atoms are same. So you can consider any of the atoms as a center atom. Now in the above sketch of Cl2 molecule, put the two electrons i.
A dipole moment is created in a molecule when there is a separation of charges or unequal distribution of charges across atoms. Here in the sketch of Cl2 molecule, we have assumed the right side chlorine cl2 lewis as a center atom.
Chlorine gas exists as a diatomic molecule with the chemical formula Cl 2 that belongs to the halogen group. It has a corrosive nature and is primarily used in the production of paper and clothing. Cl 2 Lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. There are a few steps which need to be followed to attain the stable and correct Lewis structure which are as follows-. The chlorine molecule contains two chlorine atoms.
The chlorine gas chemical formula is Cl2. Drawing Cl2 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct Cl2 Lewis Structure. The diatomic chlorine molecule elements come as members of the halogen family group from the periodic table. The valence electrons in the chlorine atom are seven.
Cl2 lewis
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom.
Isuzu tfr van
This step can be avoided for neutral compounds. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Hybridization in Cl 2 In the Lewis structure of Cl 2 , each chlorine atom has an sp 3 hybridization. Since there are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best Lewis structure. It is also known as electron dot structures because electrons are represented by dots in this representation. Purchase Now. A neutral molecule does not imply that all atoms in that molecule are neutral. In short, now you have to find the formal charge on both the chlorine Cl atoms present in the Cl2 molecule. There are only two atoms and they both belong to the same element, therefore, the central atom will be chlorine only. Click Start Quiz to begin! Add the contribution of side atoms and charge to the contribution of the central atom , i.
Chlorine molecule consists of two chlorine atoms.
In general, all diatomic molecules with the same atoms are non-polar in nature because they lack a dipole moment in addition to the bond. The best Lewis structure is the one in which octet rule and formal charges are satisfied. Valence shell electrons involved in bonding are known as bonding pairs of electrons bp , and those valence shell electrons that are not involved in bonding are termed as lone pairs of electrons lp. For a better understanding of the method for drawing the lewis structure of chlorine molecules, one can go through the following video. Peroxydisulfuric Acid. Contents show. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule. It tells us how electrons are organized around specific atoms in a molecule. Ethanol C2H5OH lewis structure. View Test Series. It is a theoretical concept.

0 thoughts on “Cl2 lewis”