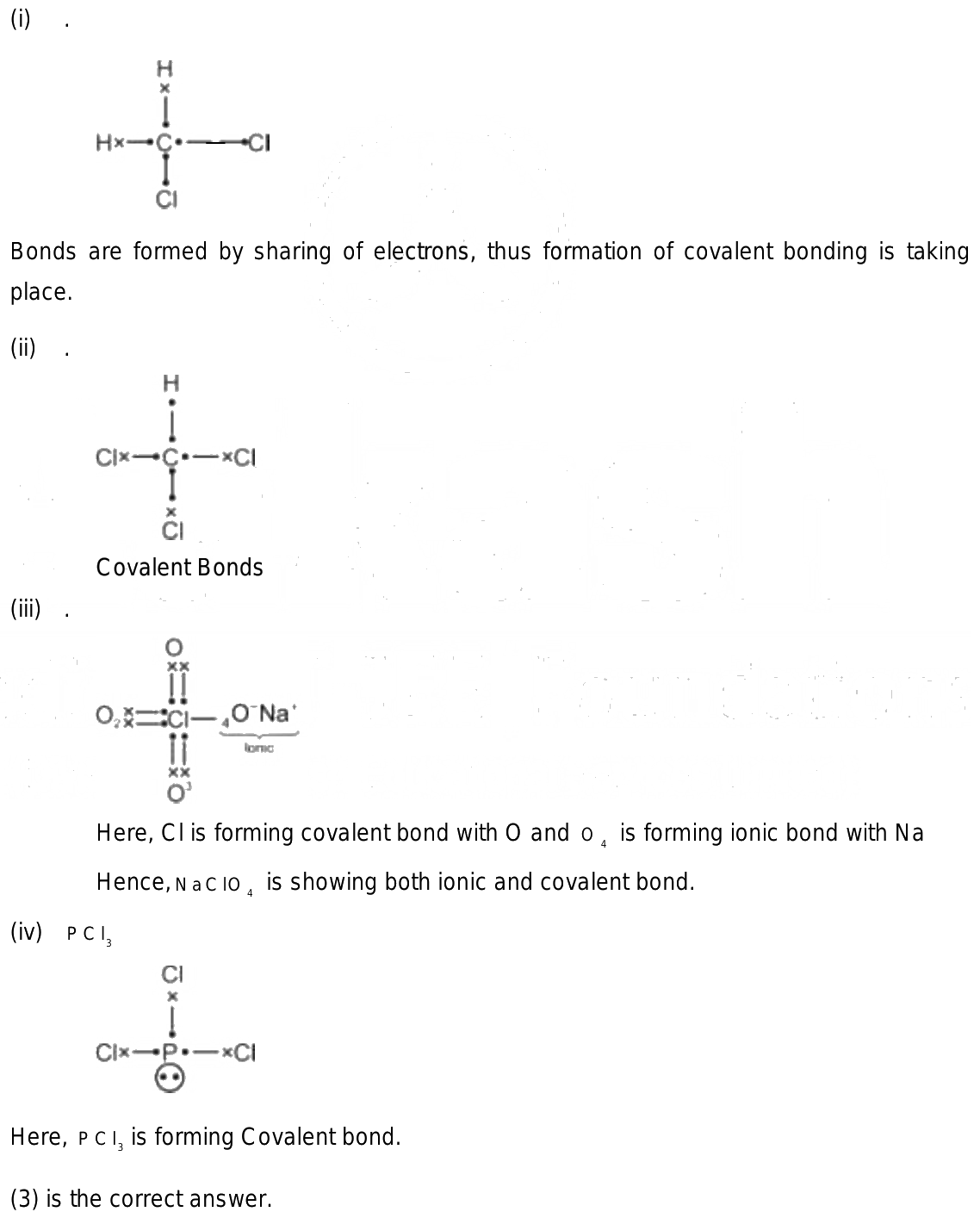
Compound containing both ionic and covalent bonds
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bondson the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. These compounds contain polyatomic ions.
Last updated on Jan 7, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam.
Compound containing both ionic and covalent bonds
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals. Less often, the cation is polyatomic. Sometimes nonmetals bond to form a cation with enough electronegativity difference from the anion to form an ionic bond! Here are examples of compounds with both ionic and covalent bonds. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. A covalent bond involves atoms sharing electrons. In pure covalent bonds, this sharing is equal. In polar covalent bonds, the electron spends more time with one atom than the other. For example, in potassium cyanide KCN , the carbon C and nitrogen N are both nonmetals, so they share a covalent bond. The potassium atom K is a metal, so it bonds to the nonmetallic anion via an ionic bond. X-ray diffraction of KCN crystals verifies this arrangement. The potassium ions are separate from the bonded carbon and nitrogen ions that form the cyanide anion.
Patna Civil Court Peon. SSC Exams. Telangana High Court Record Assistant.
.
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bonds , on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. These compounds contain polyatomic ions. Many of these compounds contain a metal, a nonmetal, and also hydrogen. However, other examples contain a metal joined via an ionic bond to covalently bonded nonmetals. Here are examples of compounds that exhibit both types of chemical bonding:. In ammonium sulfide, the ammonium cation and the sulfide anion are ionically bonded together, even though all of the atoms are nonmetals. The electronegativity difference between ammonium and the sulfur ion allows for an ionic bond. At the same time, the hydrogen atoms are covalently bonded to the nitrogen atom.
Compound containing both ionic and covalent bonds
If you know the chemical formula of a compound, you can predict whether it contains ionic bonds, covalent bonds, or a mixture of bond types. Nonmetals bond to each other via covalent bonds while oppositely charged ions, such as metals and nonmetals, form ionic bonds. Compounds which contain polyatomic ions may have both ionic and covalent bonds. But, how do you know if a compound is ionic or covalent just by looking at a sample? This is where the properties of ionic and covalent compounds can be useful. Because there are exceptions, you need to look at several properties to determine whether a sample is ionic or covalent, but here are some characteristics to consider:. Most ionic compounds have a metal as the cation or first part of their formula, followed by one or more nonmetals as the anion or second part of their formula.
Slugger o toole
Airforce Group X. RPSC Programmer. Rajasthan Animal Attendant. Properties of Ionic and Covalent Compounds. FCI Assistant Grade 3. The covalent bonds could occur in a polyatomic ion in either the cation or the anion. RPF SI. India Post Mail Guard. Karnataka Bank PO. Osteoclasts are associated with which of the following? Metallic Bond: Definition, Properties, and Examples. Bihar Police Fireman. RBI Assistant.
As you have learned, ions are atoms or molecules bearing an electrical charge. A cation a positive ion forms when a neutral atom loses one or more electrons from its valence shell, and an anion a negative ion forms when a neutral atom gains one or more electrons in its valence shell.
Maharashtra State Excise Jawan. GPSC Class SBI SO. Heat is evolved during. RRB Technician. Punjab Police SI. Odisha Police Driver. RBI Grade B. ISRO Assistant. CWC Assistant Engineer. FCI JE. MP Vyapam Group 5. South Indian Bank Clerk.

Bravo, is simply excellent phrase :)
I am sorry, that I interrupt you, but, in my opinion, there is other way of the decision of a question.
It is remarkable, the useful message