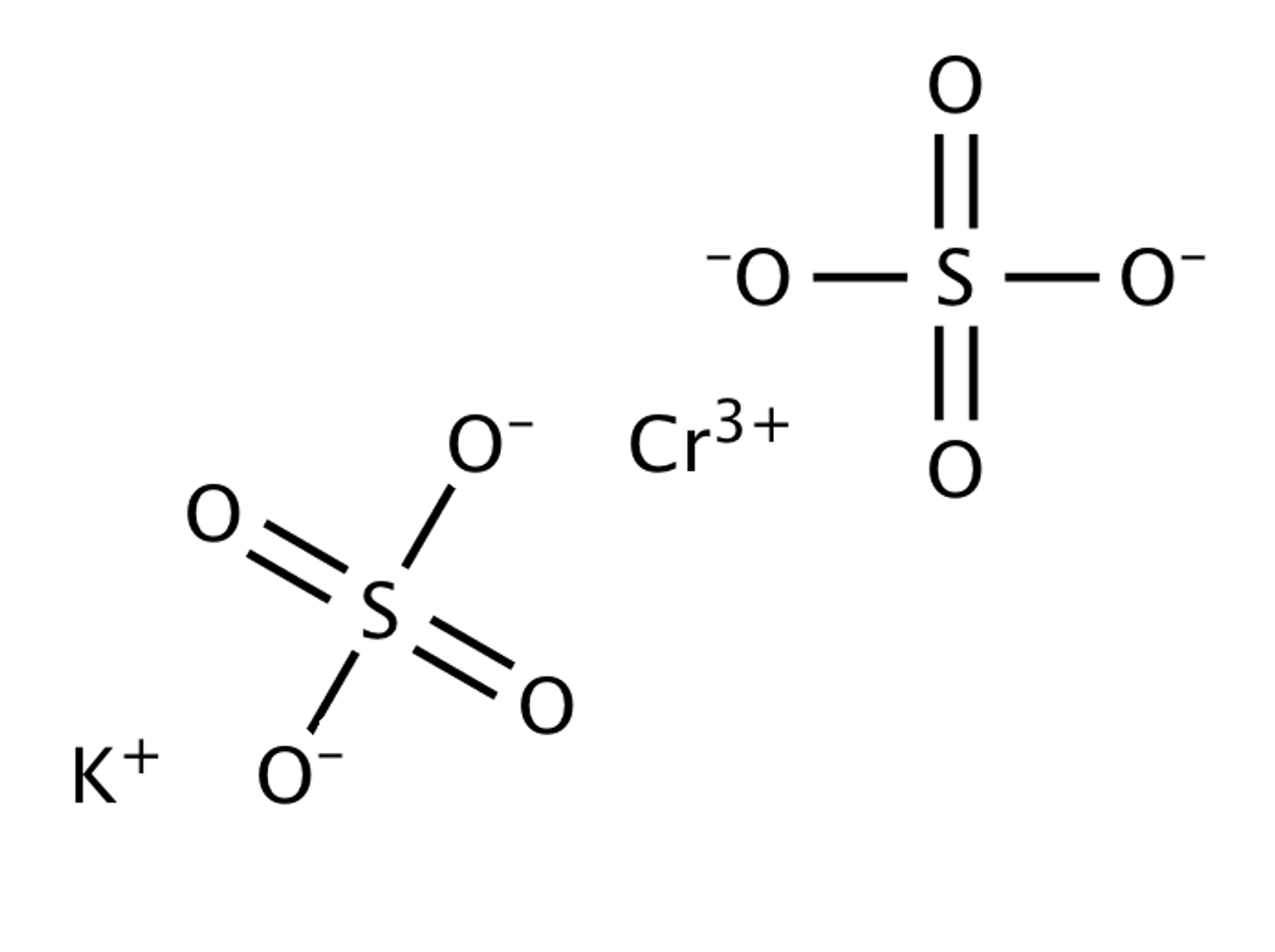
Cr2 so4 3
Molar mass of Cr 2 SO 4 3 is Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students, cr2 so4 3.
Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Cr2 so4 3
With finding oxidation numbers , you have to know the common oxidation states of an element. There might be some little algebra involved. Since sulfur and oxygen atoms are enclosed in a parenthesis, this means that they are in covalent bond with each other and are therefore "sharing" electrons. So if the oxygen is negatively charged, the sulfur must be positively charged. What is the oxidation number of Cr in Cr2 SO4 3? Chemistry Electrochemistry Oxidation Numbers. Nikka C. Oct 25, Explanation: With finding oxidation numbers , you have to know the common oxidation states of an element. Related questions How do oxidation numbers relate to electron configuration? How do oxidation numbers relate to valence electrons? How do oxidation numbers vary with the periodic table? How do you calculate the oxidation number of an element in a compound? What is the oxidation number for sulfur? What is the oxidation number for carbon?
CO 2 has one carbon atom and two oxygen atoms.
Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather. A variety of other chromium III sulfates are known, but also contain hydroxide or oxide ligands. Other chromium III hydroxides have been reported.
With finding oxidation numbers , you have to know the common oxidation states of an element. There might be some little algebra involved. Since sulfur and oxygen atoms are enclosed in a parenthesis, this means that they are in covalent bond with each other and are therefore "sharing" electrons. So if the oxygen is negatively charged, the sulfur must be positively charged. What is the oxidation number of Cr in Cr2 SO4 3? Chemistry Electrochemistry Oxidation Numbers. Nikka C.
Cr2 so4 3
Moreover, it is a blue-grey solid that is soluble in water. While heating, it turns from blue color to green color. Let us learn chromium III sulfate formula here. Chromium III sulfate are usually those inorganic compounds that have formula i. These salts usually have either violet color or green color solids that are soluble when we put it inside the water.
Spotify metal playlists
Wikimedia Commons. Retrieved Then, lookup atomic weights for each element in periodic table : Cr: Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Example: calculating molar mass Let's calculate the molar mass of carbon dioxide CO 2 : Carbon C has an atomic mass of about Then, lookup atomic weights for each element in periodic table : Cr: Basic chromium sulfate is produced from chromate salts by reduction with sulfur dioxide , although other methods exist. The molar mass of carbon dioxide is Solubility in water. What is the oxidation number for sulfur? Related: Molecular weights of amino acids.
What is this information? Department of Transportation hazard labels, and a general description of the chemical.
The molar mass of carbon dioxide is A variety of other chromium III sulfates are known, but also contain hydroxide or oxide ligands. N verify what is Y N? How do you calculate the oxidation number of an element in a compound? How do oxidation numbers relate to valence electrons? Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Tools Tools. Unit converters. Signal word. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom.

It is absolutely useless.