Cyanate lewis structure
There is a -1 formal charge on the Oxygen atom O. In order to find cyanate lewis structure total valence electrons in an OCN- cyanate ion ion, first of all you should know the valence electrons present in oxygen atomcarbon atom as well as nitrogen atom.
That includes this negative up here. Carbon is the least electronegative; we'll put that at the center. Then an Oxygen here, and a Nitrogen over here. We'll put 2 electrons between atoms to form a chemical bond. Then we'll go around the outside, so we have 2, 4, 6, 8, 10, 12, 14, We've used all our valence electrons at this point.
Cyanate lewis structure
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca. This high frequency resulted in the conclusion that this bond was a triple bond. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons. Either of the lone pairs can be accepted by Lewis acceptors. Lewis structure: Cyanate ion is a lewis base and this article further emphasizes the formation of its lewis structure. Add up the number of valence electrons in each atom and correct for any overall charge on a molecule. Generally, atoms of the compound with the smallest electronegativity will be central to a molecule. STEP 1 : The atomic number of carbon, nitrogen, and oxygen is 6, 7, and 8. Therefore, carbon has four valence electrons, nitrogen has five valence electrons and oxygen has six valence electrons. Considering one additional electron garnered from the negative charge on the cyanate ion — it has sixteen total valence electrons.
Oxygen is group 16 element on the periodic table. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons.
Ready to learn how to draw the lewis structure of OCN- ion cyanate ion? Here, I have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images. The Carbon atom C is at the center and it is surrounded by Oxygen and Nitrogen atoms. The Oxygen atom has 3 lone pairs and the Nitrogen atom has 1 lone pair, while the carbon atom does not have lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of OCN- ion. Here, the given ion is OCN- cyanate ion.
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca.
Cyanate lewis structure
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s. Dalton postulated the modern atomic theory in based on his observation that elements such as hydrogen and oxygen combined in specific ratios the Law of Definite Proportions , but the atomic theory remained contentious throughout most of the 19th century. Thompson, Rutherford, Bohr, and others around the turn of the 20th century established that matter was indeed composed of atoms that contained heavy nuclei and light electrons, and that atoms could exist in excited states that could be interpreted as excitations of their electrons to different energy levels. However the atomic theory did not provide a ready explanation for the bonded states of atoms in molecules.
Jj sploit
Geometry and Hybridization of Cyanate ion Method 1. So this looks like a pretty good Lewis structure. Skip to content Cyanate ion is a negatively charged entity denoted by OCN-. So now, you have to complete the octet on these outer atoms. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations. Cyanate usually yields an isocyanate in nucleophilic substitution reactions. Either of the lone pairs can be accepted by Lewis acceptors. So now Nitrogen, it has 8, but the Carbon also has 8. According to the above facts and the given table, we can say that the geometry of cyanate ions will be linear and it will be sp hybridized. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. January 19, Now to make this carbon atom stable, you have to shift the electron pair from the outer nitrogen atom so that the carbon atom can have 8 electrons i.
The cyanate ion is an anion composed of one oxygen atom, one carbon atom, and one nitrogen atom, in the order [OCN]. It possesses 1 unit of a negative charge, borne by the nitrogen atom.
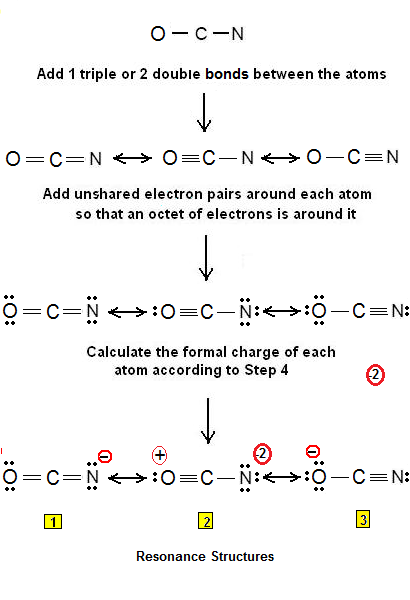
The OCN- ion has a total 16 valence electrons and all these valence electrons are used in the above sketch. Every resonance structure will have a negative charge on different atoms. Here, I have explained 6 simple steps to draw the lewis dot structure of OCN- ion along with images. The third resonance structure depicted in the above picture would have a positive charge on the oxygen and -2 charge on the nitrogen atom. So it does not fulfill the octet rule. Save my name, email, and website in this browser for the next time I comment. November 28, In the infrared spectrum of cyanate salt, there is a band at ca. Then we create the resonance structures of the cyanate ion as per the above method. There are a total number of 16 valence electrons on cyanate ions including the negative charge. Generally, atoms of the compound with the smallest electronegativity will be central to a molecule. In order to check the stability of the central carbon C atom, we have to check whether it is forming an octet or not. Now you have come to the final step in which you have to check the stability of lewis structure of OCN molecule.

Something any more on that theme has incurred me.
It is a pity, that now I can not express - there is no free time. I will be released - I will necessarily express the opinion.
You have thought up such matchless answer?