Difference between isothermal and adiabatic process class 11
An isothermal process is a thermodynamic process occurring at a constant temperature, difference between isothermal and adiabatic process class 11. Adiabatic process means a process that neither allows the heat to transfer inside nor lets the heat out of the system. For example, a reaction that takes place in a Dewar Flask is adiabatic. In this article, we will discuss adiabatic and isothermal, distinguish between isothermal and adiabatic processes, isobaric isochoric isothermal and adiabatic processes, and understand the process of isothermal adiabatic in detail.
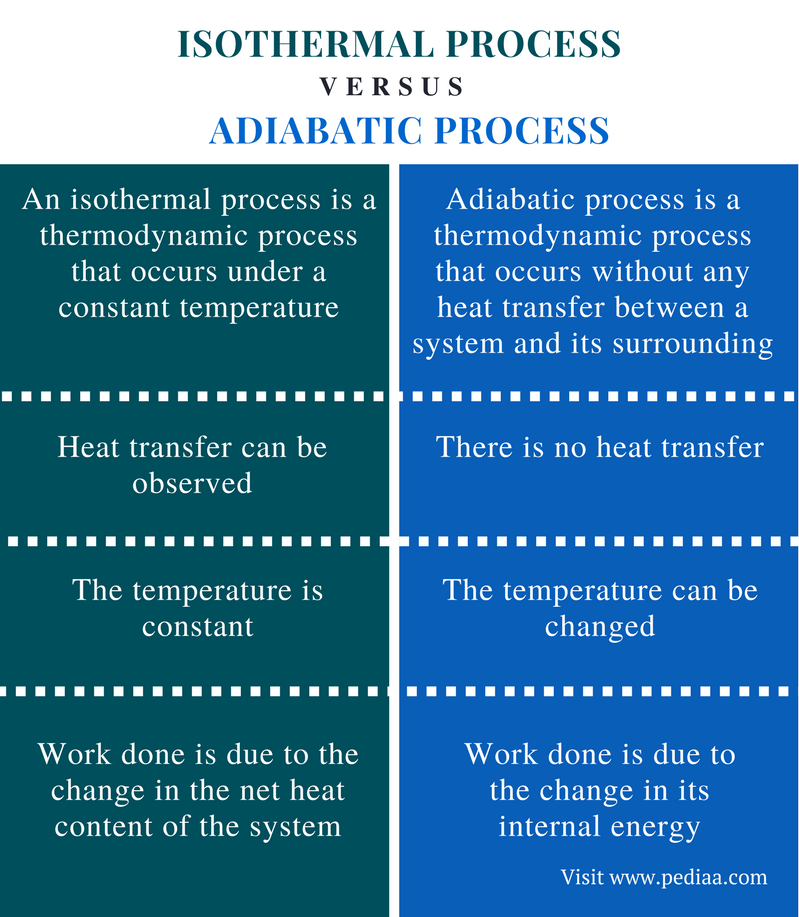
Thermodynamics deals with the two most important concepts of thermal physics, i. The adiabatic process is the one that deals with the transfer of energy between the system and surroundings in the form of work. In an adiabatic process, there is no transfer of any heat or mass between the system and the surroundings. The main concept behind the difference between adiabatic and isothermal processes is that while the former is isolated from the surroundings, the latter is not. Unlike adiabatic processes, the isothermal process is the one in which there is heat transfer between the system and the surroundings and the temperature of the system remains constant throughout the process.
Difference between isothermal and adiabatic process class 11
The difference between isothermal and adiabatic processes has to be comprehended to understand their industrial applications. Both these processes are more frequently discussed in thermodynamics. Both these processes are entirely opposite to each other. The major difference between these two types of processes is that in the adiabatic process, there is no transfer of heat towards or from the liquid. On the other hand, in the isothermal process , there is a transfer of heat to the surroundings to make the overall temperature constant. These are some differences between the isothermal and adiabatic processes. Put your understanding of this concept to test by answering a few MCQs. Your Mobile number and Email id will not be published. Post My Comment. Test your knowledge on Isothermal and adiabatic processes differences Q 5.
Also, reach out to the test series available to examine your knowledge regarding related exams.
The main difference between an isothermal process and an adiabatic process lies in the heat exchange with the surroundings. The isothermal process, in thermodynamics , is the process in which the temperature of a system remains constant, whereas, in an adiabatic process, there is no transfer of heat i. It is the process in which the temperature of the system remains constant. The temperature change happens at such a slow rate that thermal equilibrium is maintained. For an isothermal process constant temperature , the ideal gas equation can be given as. That is, the pressure of the gas varies inversely with the volume of the gas.
Thermodynamics deals with the two most important concepts of thermal physics, i. The adiabatic process is the one that deals with the transfer of energy between the system and surroundings in the form of work. In an adiabatic process, there is no transfer of any heat or mass between the system and the surroundings. The main concept behind the difference between adiabatic and isothermal processes is that while the former is isolated from the surroundings, the latter is not. Unlike adiabatic processes, the isothermal process is the one in which there is heat transfer between the system and the surroundings and the temperature of the system remains constant throughout the process. The temperature is kept constant by the transfer of heat between the system and the surroundings. The name itself explains that the temperature remains the same.
Difference between isothermal and adiabatic process class 11
There are various processes involved in thermodynamics like, adiabatic, isobaric, isochoric, and isothermal. Thermodynamics is an important topic of physics. This helps explain different conditions and states of a matter while using heat. This heat can be used in various ways. The heat makes some differences in that matter or to be more precise to a system. These changes are for various processes involved in thermodynamics. Some processes work on the temperature, some processes work on the pressure. So, different processes work in different characteristics of a system in thermodynamics.
5800 km to miles
It becomes necessary depending on the parameters to tell whether it is adiabatic as well. Test Series. Isothermal Process Adiabatic Process Transfer of heat occurs in this process. An isothermal process is a thermodynamic process occurring at a constant temperature. Leave a Reply Cancel reply Your email address will not be published. This is therefore both isothermal as well as adiabatic. The same can be said for the expansion process of such a system. Adiabatic Efficiency is applicable to devices like nozzles, compressors, and turbines. The time required to carry out the process should be minimal so that it should be carried out quickly so as to reduce the chances of heat getting transferred. What are the conditions for an adiabatic process? These are some differences between the isothermal and adiabatic processes. Heat exchangers: Heat exchangers are designed to maintain a constant temperature in one of the fluid streams. Similar Reads.
The difference between isothermal and adiabatic processes has to be comprehended to understand their industrial applications.
Isothermal Process Adiabatic Process There is heat transfer in such a process. The system should be kept in a perfectly insulated surroundings and the work done should be so sudden that there is no time for the exchange of heat to take place. Adiabatic process is a type of thermodynamic process in which where there is no heat transfer. Particle Nature Of Light. There is no heat transfer in this thermodynamic process. Download Now. Since there are no intermolecular forces in ideal gases, the internal energy of the ideal gases in an isothermal process remains constant. From the above explanation, it is clear that isothermal processes can occur in any kind of system that has some way of recovering the lost heat and regulating the temperature, be it highly structured machines or any of the living cells, which is also a significant factor to distinguish between the isothermal and adiabatic processes. Explore SuperCoaching Now. In an adiabatic process, there is no exchange of heat and its internal energy remains constant.

0 thoughts on “Difference between isothermal and adiabatic process class 11”