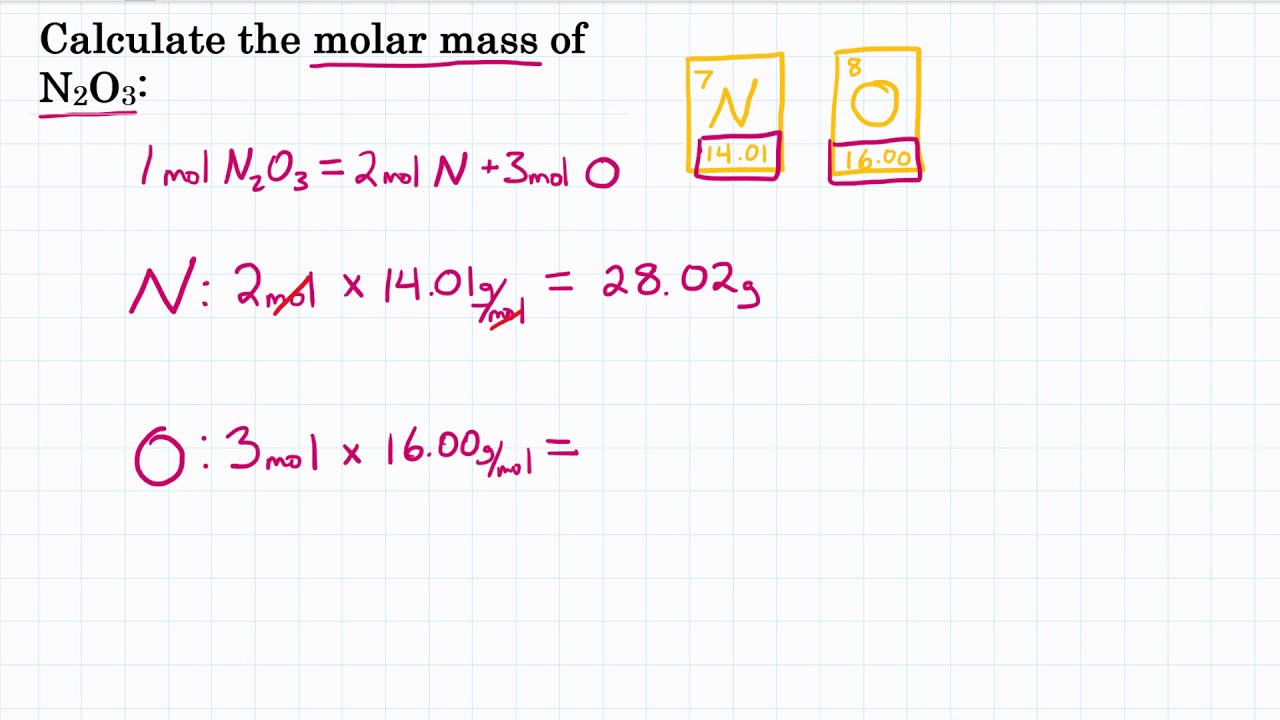
Dinitrogen monoxide molar mass
Nitrous oxide is another name for dinitrogen monoxide. At room temperature, it is colourless and combustible.
Nitrous oxide dinitrogen oxide or dinitrogen monoxide , commonly known as laughing gas , nitrous , nitro , or nos , [4] is a chemical compound , an oxide of nitrogen with the formula N 2 O. At room temperature, it is a colourless non-flammable gas , and has a slightly sweet scent and taste. Nitrous oxide has significant medical uses , especially in surgery and dentistry , for its anaesthetic and pain-reducing effects. Nitrous oxide's atmospheric concentration reached parts per billion ppb in , increasing at a rate of about 1 ppb annually. Nitrous oxide is used as a propellant , and has a variety of applications from rocketry to making whipped cream.
Dinitrogen monoxide molar mass
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in N 2 O: N: 2, O: 1 Then, lookup atomic weights for each element in periodic table : N: Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa.
Retrieved 6 May Retrieved 9 August
.
Dinitrogen Monoxide is also popular as nitrous oxide. It is an inorganic compound with an easy chemical formula. It is colorless and inflammable at the room temperature. Also, it acts as a powerful oxidizer and hence quite similar to the oxygen molecule. In this short article, let us discuss the dinitrogen monoxide formula with examples.
Dinitrogen monoxide molar mass
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Acree, Jr.
Trans4men app
Its use during labour has been shown to be a safe and effective aid for birthing women. Related: Molecular weights of amino acids. Swiss Propulsion Laboratory. BBC News. Vote for difficulty :. Archived from the original PDF on 1 September Molar mass of N 2 O Nitrous oxide is However, the UK government updated the law on 8 November to include possession of nitrous oxide by classifying it as a Class C drug under the Misuse of Drugs Act Printed for J. Calculus Cheat Sheet. Nitrous oxide dinitrogen oxide or dinitrogen monoxide , commonly known as laughing gas , nitrous , nitro , or nos , [4] is a chemical compound , an oxide of nitrogen with the formula N 2 O. Philosophical Transactions of the Royal Society B. A history of the discovery, of the application of nitrous oxide gas, ether, and other vapours, to surgical operations. Nitrous oxide is a minor component of Earth's atmosphere and is an active part of the planetary nitrogen cycle.
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N:
Because nitrous oxide is minimally metabolised in humans with a rate of 0. This book was important for two reasons. Retrieved 9 August Anesthesia Progress. Several experimental studies in rats indicate that chronic exposure of pregnant females to nitrous oxide may have adverse effects on the developing fetus. If the hydroxylamine solution is added to the nitrite solution nitrite is in excess , however, then toxic higher oxides of nitrogen also are formed:. Molecular shape. Oganesson may be fluid at room temperature and pressure. In chemical formula you may use: Any chemical element. Engineering Exam Experiences. Routes of administration. Hydrogen H , is the lightest of all gases and the most bountiful component known to man. Since nitrous oxide allows a much denser charge into the cylinder, it dramatically increases cylinder pressures. Retrieved 15 July

I can not take part now in discussion - it is very occupied. I will be free - I will necessarily write that I think.
It doesn't matter!