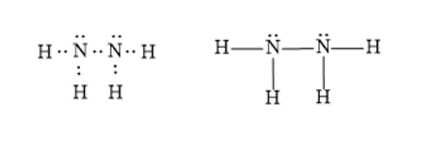
Dinitrogen tetrahydride
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet. How it is incorporated into the biosphere is complicated by its intrinsic inertness. For example, biological nitrogen fixation takes N-2 and converts it into ammonia using various nitrogenase enzymes, whereas industrial nitrogen fixation converts N-2 and H-2 to NH3 using heterogeneous iron or ruthenium surfaces. In both cases, dinitrogen tetrahydride, the processes are dinitrogen tetrahydride.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together.
Dinitrogen tetrahydride
E-mail: ynishiba sogo. A dinitrogen-bridged dimolybdenum-tetrachloride complex is prepared and reduced with Super-Hydride LiBHEt 3 to afford the corresponding dimolybdenum-dinitrogen complex together with the formation of molecular dihydrogen. This reaction proceeds via the ligand exchange of the coordinated dihydrogen generated in situ with molecular dinitrogen. As the next stage of the previous work, we have focused on the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen at ambient temperature and pressure. To achieve the catalytic formation of ammonia as the next nitrogen fixation, in place of the Haber—Bosch process, 7 the ruthenium—hydride species should reduce the high oxidative tungsten species to regenerate the corresponding tungsten—dinitrogen complex. However, unfortunately, the tungsten species can not be reduced with the ruthenium—hydride species or with other hydride species such as LiBHEt 3. As the first stage of the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen under mild reaction conditions, we envisaged the reaction of the high oxidative molybdenum complexes with hydride species to regenerate the corresponding dinitrogen complexes as starting catalytic species. In this reaction, the high oxidative molybdenum complexes can be reduced into the corresponding dinitrogen complexes, where the ligand exchange of the coordinated dihydrogen with molecular dinitrogen may be involved as a key step to regenerate the corresponding dinitrogen complexes. Herein, we describe the preparation of the dinitrogen-bridged dimolybdenum-tetrachloride complex bearing a PNP -type pincer ligand and the reduction of the dimolybdenum-tetrachloride complex with Super-Hydride LiBHEt 3 to afford the corresponding molybdenum-dinitrogen complex together with the formation of molecular dihydrogen. No informative data on the structure of 2 were obtained from its NMR spectra due to the paramagnetism. A more detailed molecular structure of 2 is determined by X-ray crystallographic study Fig.
In other projects.
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Myron Cummings Modified over 8 years ago. NCl3 nitrogen trichloride b.
Hydrazine is a molecule of two singly-bonded nitrogen atoms and four peripheral hydrogen atoms. In its anhydrous form, it is a colourless, toxic irritant and sensitiser, which damages the central nervous system, producing symptoms as extreme as tumours and seizures. The pungent smell of hydrazine is not unlike that of ammonia, and it is so powerful a reducing agent that it is highly explosive. Considering this, it seems strange that around , metric tonnes of the stuff are manufactured worldwide every year. But hydrazine does influence our everyday lives. It keeps us warm, clothes and feeds us, can save our lives and even take us to the moon. In a way, it can even turn back time. The most common use of hydrazine is to make foaming agents like azodicarbonamide. When azodicarbonamide is bubbled through a liquid polymer precursor, it thermally decomposes to nitrogen, carbon dioxide, carbon monoxide and ammonia. These gases form bubbles in the liquid, which then polymerises to leave a lightweight, foamy plastic.
Dinitrogen tetrahydride
Acid halides are named by identifying first the acyl group and then the halide. As described in Section To keep things interesting, however, IUPAC recognizes eight exceptions for which a - yl rather than an - oyl ending is used: formic formyl , acetic acetyl , propionic propionyl , butyric butyryl , oxalic oxalyl , malonic malonyl , succinic succinyl , and glutaric glutaryl. Symmetrical anhydrides of unsubstituted monocarboxylic acids and cyclic anhydrides of dicarboxylic acids are named by replacing the word acid with anhydride. Unsymmetrical anhydrides—those prepared from two different carboxylic acids—are named by listing the two acids alphabetically and then adding anhydride.
Samsung tv instruction manual
Related nitrogen oxides. This hot nitrogen dioxide is expanded through a turbine, cooling it and lowering the pressure, and then cooled further in a heat sink, causing it to recombine into nitrogen tetroxide at the original molecular weight. Its molar mass is Article Talk. Interactive image. Gmelin Reference. Oxygen compounds. Nitrogen tetroxide is made by the catalytic oxidation of ammonia : steam is used as a diluent to reduce the combustion temperature. In early , research on the usability of dinitrogen tetroxide as an oxidizing agent for rocket fuel was conducted by German scientists, although the Germans only used it to a very limited extent as an additive for S-Stoff fuming nitric acid. Publishing as Benjamin. Journal of the American Chemical Society.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide.
So we developed a line of study tools to help students learn their way. J: Prentice Hall. Upper Saddle River, N. External SDS. At first, a dinitrogen-bridged dimolybdenum-tetrahydride complex A may be formed from the reaction of dimolybdenum-tetrachloride complex 2 with LiBHEt 3 and then the dinitrogen-bridged dimolybdenum-tetrahydride complex A is converted into the dinitrogen-bridged dimolybdenum-bis dinitrogen complex C via a dinitrogen-bridged dimolybdenum-bis dihydrogen complex B as a key intermediate. Wikimedia Commons has media related to Dinitrogen tetroxide. Pedro Paulet , a Peruvian polymath , reported in that he had experimented in the s with a rocket engine that used spring-loaded nozzles that periodically introduced vaporized nitrogen tetroxide and a petroleum benzine to a spark plug for ignition, with the engine putting out pulsating explosions per minute. Pedro Paulet , a Peruvian polymath , reported in that he had experimented in the s with a rocket engine that used spring-loaded nozzles that periodically introduced vaporized nitrogen tetroxide and a petroleum benzine to a spark plug for ignition, with the engine putting out pulsating explosions per minute. More Than Just We take learning seriously. The balanced equation for this reaction is:. CCDC This was due to a switch accidentally left in the wrong position, which allowed the attitude control thrusters to fire after the cabin fresh air intake was opened, allowing NTO fumes to enter the cabin.

Absolutely with you it agree. In it something is also to me it seems it is excellent thought. Completely with you I will agree.
In it something is. Thanks for an explanation, the easier, the better �