Do ionic compounds dissolve in water
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
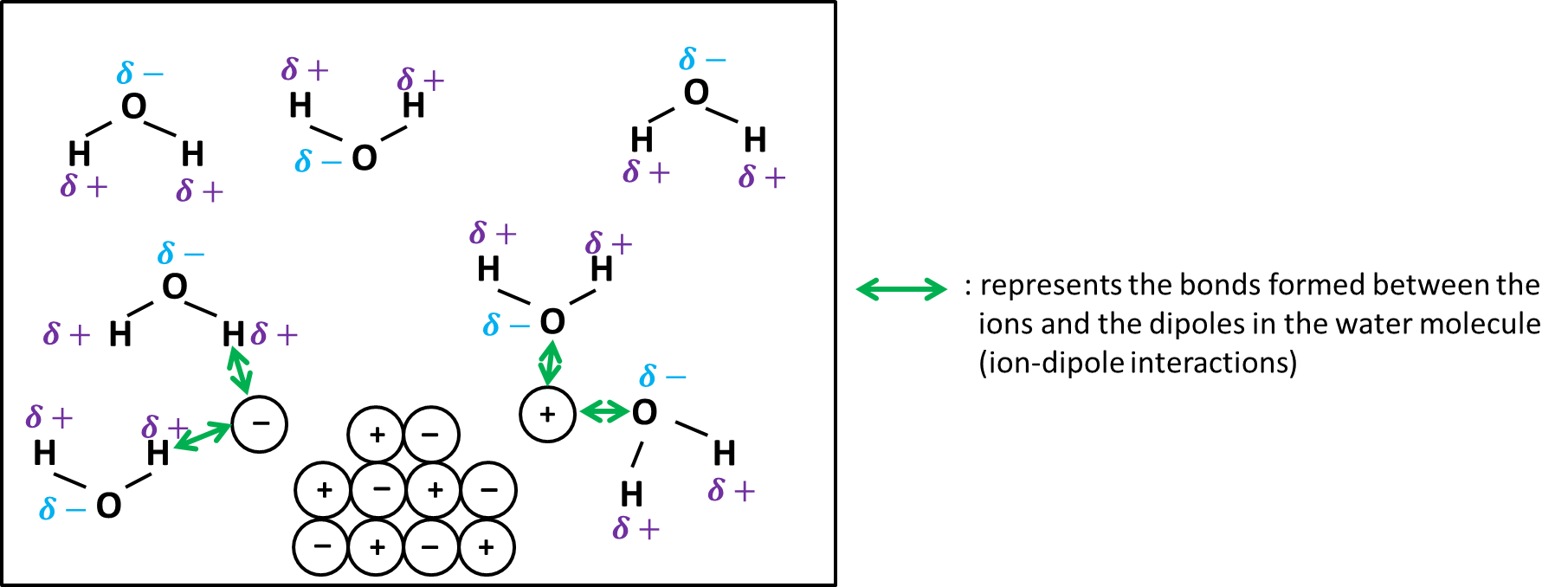
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. The negative ions have several water molecules around them, all with their "H" atoms close to the negative ion.
Do ionic compounds dissolve in water
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. But how exactly do solutions form? Why is it that oil and water will not form a solution, and yet vinegar and water will? Why could we dissolve table salt in water, but not in vegetable oil? The reasons why solutions will form will be explored in this section, along with a discussion of why water is used most frequently to dissolve substances of various types. In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent. This maximum amount is specified as the solubility of the solute. It is usually expressed in terms of the amount of solute that can dissolve in g of the solvent at a given temperature. These solubilities vary widely. NaCl can dissolve up to When the maximum amount of solute has been dissolved in a given amount of solvent, we say that the solution is saturated with solute. When less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturated. These terms are also qualitative terms because each solute has its own solubility. A solution of 0.
In solution, each water molecule acts like a tiny magnet that creates a force of attraction on the ions in the solute.
Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. Salts, including sodium chloride NaCl — table salt —are the best-known examples of ionic compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining. The resultant ionic solution becomes an electrolyte, which means it can conduct electricity.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Solutions of weak electrolytes still contain ions, put the extent to which they form ions is solution is much lower than for strong electrolytes, so they do not conduct as well. Solutions of nonelectrolytes do not contain any ions and therefore do not conduct electricity. The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an important role in the dissolution of ionic compounds in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them.
Do ionic compounds dissolve in water
Ionic compounds usually dissociate in water because water is a polar molecule. When the substance is placed in water, the water molecules pull the positive and negative ions apart from each other. The water molecules attract the ions from the surface of the solid. As a result, the ions move into the water. This shell of water molecules surrounding the ions in solution stabilizes them and reduces the attractions of the positive and negative ions for each other. You can look up here something about ionic bonding. Water is a covalent polar compound it has positive and negative poles. Also, ionic compound tend to form complex lattice networks and structures see the picture.
Floran starbound
Learning Objectives Define and give examples of electrolytes. If the hydration energy of the compound is lesser than the lattice energy, the compound will not dissolve. Feb 25, How is a Water Molecule Like a Magnet? The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. All nitrates, chlorates, perchlorates and acetates. Most compounds containing the bromide ion are soluble, but lead II is an exception. One of the K superscript plus purple spheres is surrounded by four of the red and white clusters. Explain how water molecules attract ionic solids when they dissolve in water. How to Test for Hydrochloric Acid. Solutions of electrolytes contain ions that permit the passage of electricity.
A simple ionic compound, such as sodium chloride NaCl consists of a sodium cation and a chloride anion. Because these are oppositely charge ions, they are strongly attracted to each other.
CaCO 3. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining. Here's a video that shows the process in action. Question cd Most compounds containing the bromide ion are soluble, but lead II is an exception. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. He began writing online in , offering information in scientific, cultural and practical topics. They are all gases at standard pressure. In each case, the wire leads from the wall to the beaker and is split resulting in two ends. We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. How to Test for Hydrochloric Acid.

0 thoughts on “Do ionic compounds dissolve in water”