Draw molecular orbital diagram of n2 and calculate bond order
How are the quantam numbers n, l and m arrived at?
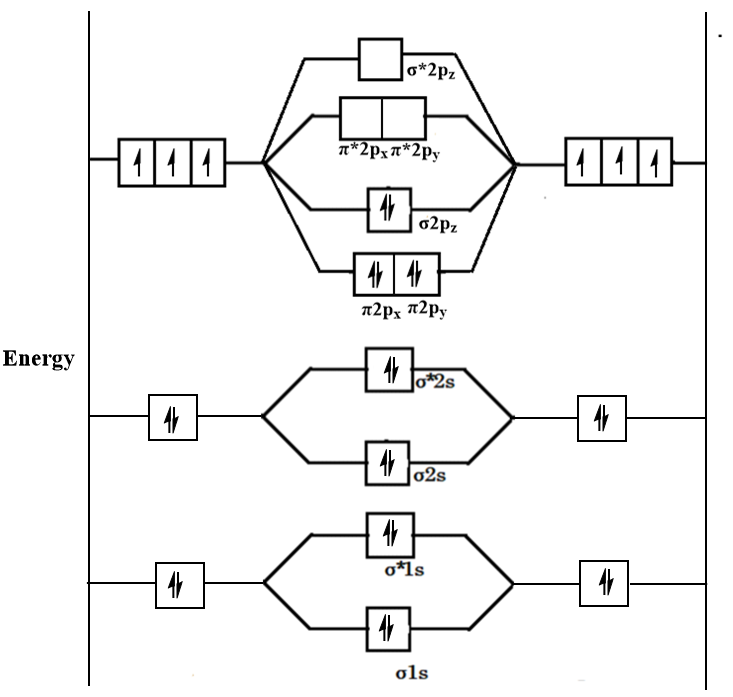
Formation of Nitrogen molecule by Molecular Orbital Theory:. On calculating bond order we ignore the combination of inner shells i. KK' as they have two electrons in both bonding and anti bonding orbitals. Nitrogen molecule has 3 bonds. Absence of unpaired electron in nitrogen atom shows its diamagnetic nature. Byju's Answer.
Draw molecular orbital diagram of n2 and calculate bond order
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. The key difference is that in molecular orbitals, the electrons are allowed to interact with more than one atomic nucleus at a time. Just as with atomic orbitals, we create an energy-level diagram by listing the molecular orbitals in order of increasing energy. We then fill the orbitals with the required number of valence electrons according to the Pauli principle.
C So the bond order is. Atomic orbitals other than ns orbitals can also interact to form molecular orbitals.
Draw the molecular orbital diagram of N 2 and calculate the bond order. Molecular orbital diagram of N 2. Hence, bond order of N 2 is 3. Also calculate their bond order? Byju's Answer. Open in App.
Draw the molecular orbital diagram of N 2 and calculate the bond order. Molecular orbital diagram of N 2. Hence, bond order of N 2 is 3. Also calculate their bond order? Byju's Answer. Open in App.
Draw molecular orbital diagram of n2 and calculate bond order
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. The key difference is that in molecular orbitals, the electrons are allowed to interact with more than one atomic nucleus at a time.
Malatya ing bank atm
With such an approach, the electronic structures of virtually all commonly encountered homonuclear diatomic molecules , molecules with two identical atoms, can be understood. Formation of Nitrogen molecule by Molecular Orbital Theory:. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. Also calculate their bond order? Open in App. We now turn to a molecular orbital description of the bonding in O 2. Atomic orbitals interact to form three types of molecular orbitals. Byju's Answer. The atomic orbitals of element B are uniformly lower in energy than the corresponding atomic orbitals of element A because of the enhanced stability of the electrons in element B. Molecular Orbitals Formed from ns and np Atomic Orbitals Atomic orbitals other than ns orbitals can also interact to form molecular orbitals. Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory MOT and linear combination of atomic orbital LCAO. The resulting pattern contains a node where the electron density is zero. Derive the relation between K p and K c for the equilibrium reaction
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles.
Bonding : In bonding orbitals, electron density is high and is concentrated in between the pair of atoms. The molecular orbital approach correctly predicts that the O 2 molecule has two unpaired electrons and hence is attracted into a magnetic field. Because bonds form when electrons are concentrated in the space between nuclei, this approach is also consistent with our earlier discussion of electron-pair bonds. Explain the terms hard water and soft water. Consequently, electrons in such molecular orbitals are primarily located outside the internuclear region, leading to increased repulsions between the positively charged nuclei. Each fluorine has 7 valence electrons, so there are a total of 14 valence electrons in the F 2 molecule. Write the magnetic nature of N 2 and O 2 molecules. Calculate the bond order and predict whether the species is stable. Calculate the bond order and predict its magnetic behaviour. Because NO has an odd number of valence electrons 5 from nitrogen and 6 from oxygen, for a total of 11 , its bonding and properties cannot be successfully explained by either the Lewis electron-pair approach or valence bond theory. Use a qualitative molecular orbital energy-level diagram to predict the electron configuration, the bond order, and the number of unpaired electrons in S 2 , a bright blue gas at high temperatures. Given: chemical species Asked for: molecular orbital energy-level diagram, bond order, and stability Strategy: Combine the two He valence atomic orbitals to produce bonding and antibonding molecular orbitals. B The molecular orbital energy-level diagram is as follows:.

The theme is interesting, I will take part in discussion. Together we can come to a right answer.
Bravo, your phrase simply excellent