Electrolytic cell diagram
Voltaic cells use a spontaneous chemical reaction to drive an electric current through an external circuit. These cells are important because they are the basis for the batteries that fuel modern society, electrolytic cell diagram. But they aren't the only kind of electrochemical cell.
A cell is a device capable of producing electrical energy from chemical reactions or employing electrical energy to bring about a chemical reaction. So, cells can be grouped into two major categories: one that produces electrical energy from chemical reactions and another that uses electrical energy to bring about a chemical reaction. While the former is called a galvanic or voltaic cell, the latter is an electrolytic cell. Both electrolytic and galvanic cells operate differently. The following table enumerates the key differences between electrolytic cells vs galvanic cells. However, both cells contain two half-cells for a net-redox reaction with reduction and oxidation. In both cells, oxidation occurs at the anode and reduction at the cathode.
Electrolytic cell diagram
Voltaic cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they are not the only kind of electrochemical cell. The reverse reaction in each case is non-spontaneous and requires electrical energy to occur. It is possible to construct a cell that does work on a chemical system by driving an electric current through the system. These cells are called electrolytic cells. Electrolytic cells, like galvanic cells, are composed of two half-cells--one is a reduction half-cell, the other is an oxidation half-cell. The direction of electron flow in electrolytic cells, however, may be reversed from the direction of spontaneous electron flow in galvanic cells, but the definition of both cathode and anode remain the same, where reduction takes place at the cathode and oxidation occurs at the anode. Because the directions of both half-reactions have been reversed, the sign, but not the magnitude, of the cell potential has been reversed. Electrolytic cells are very similar to voltaic galvanic cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. The main differences are outlined below:. A galvanic cell left transforms the energy released by a spontaneous redox reaction into electrical energy that can be used to perform work.
An electrolytic cell is suitable for the electrolysis of electrolytic cell diagram compounds such as water when subjected to electrolysis forms gaseous hydrogen and oxygen. Answer: 0. The electrolyte can be in the form of a solution or a fused state.
Home 9. Reactions are spontaneous and exothermic. Electrolytic Cells — convert electrical to chemical energy. Non spontaneous. By convention, anode is always of left, and cathode on right. These two are separated, connected only by a salt bride. This is the voltage generated when two different solutions come into contact with each other Salt bridge contains a concentrated solution of a strong electrolyte.
Voltaic cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they are not the only kind of electrochemical cell. The reverse reaction in each case is non-spontaneous and requires electrical energy to occur. It is possible to construct a cell that does work on a chemical system by driving an electric current through the system. These cells are called electrolytic cells. Electrolytic cells, like galvanic cells, are composed of two half-cells--one is a reduction half-cell, the other is an oxidation half-cell. The direction of electron flow in electrolytic cells, however, may be reversed from the direction of spontaneous electron flow in galvanic cells, but the definition of both cathode and anode remain the same, where reduction takes place at the cathode and oxidation occurs at the anode.
Electrolytic cell diagram
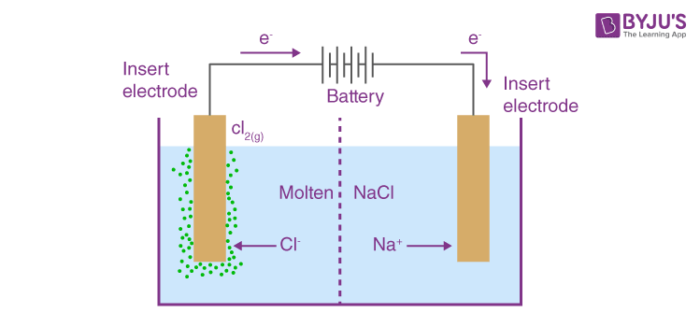
An electrolytic cell can be defined as an electrochemical device that uses electrical energy to facilitate a non-spontaneous redox reaction. Electrolytic cells are electrochemical cells that can be used for the electrolysis of certain compounds. For example, water can be subjected to electrolysis with the help of an electrolytic cell to form gaseous oxygen and gaseous hydrogen. This is done by using the flow of electrons into the reaction environment to overcome the activation energy barrier of the non-spontaneous redox reaction.
High chair cartoon
The following table enumerates the key differences between electrolytic cells vs galvanic cells. Both the electrodes are placed in a same container in the solution of molten electrolyte. The cathode has negative polarity, so cations move towards it. Ellingham Diagram. So, what is electrolysis? Michael Faraday discovered in that there is always a simple relationship between the amount of substance produced or consumed at an electrode during electrolysis and the quantity of electrical charge Q which passes through the cell. The electrolyte provides the medium for the exchange of electrons between the cathode and the anode. Electrolytic reduction of Metals from Compounds Aluminium is obtained from bauxite using an electrolytic cell. Electrolytic cells use electrical work as source of energy to drive the reaction in the opposite direction. Faraday's law of electrolysis can be stated as follows.
In galvanic cells, chemical energy is converted into electrical energy. The opposite is true for electrolytic cells. In electrolytic cells , electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis.
Because the demand for chlorine is much larger than the demand for sodium, electrolysis of aqueous sodium chloride is a more important process commercially. Voltaic cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. Calculate the volume of H 2 gas at 25 o C and 1. Then the Faraday constant can be used to find the quantity of charge. This is the voltage generated when two different solutions come into contact with each other Salt bridge contains a concentrated solution of a strong electrolyte. Answer: 0. In an electrolytic cell right , an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; only a single compartment is employed in most applications. Quantitative Aspects of Electrolysis Michael Faraday discovered in that there is always a simple relationship between the amount of substance produced or consumed at an electrode during electrolysis and the quantity of electrical charge Q which passes through the cell. The decomposition of sodium chloride is the simplest example of understanding how an electrolytic cell functions. Electrolytic Cells — convert electrical to chemical energy. According to the law, the weight of metal deposited at the cathode is directly proportional to the quantity of electricity used. Check your Knowledge: Question 1: How long will you pass a current of 4 Ampere through a solution of silver nitrate to coat a metal surface of area 50 cm 2 with a 0.

You realize, what have written?
What interesting message