Electron domains
It is very important from the onset that students understand the difference between electronic geometry and molecular geometry, electron domains. In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion VSEPR model, electron domains, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as far away as possible. Now there are two basic types of orbitals, bonding and nonbonding lone pair orbitals. The molecular orbital describes the orientation of the bonds electron domains so is citymapper london on the orientation of the bonding orbitals.
We begin by assuming a Lewis structure model for chemical bonding based on valence shell electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms share a pair of valence shell electrons between them. We know that double bonds are generally stronger and have shorter lengths than single bonds, and triple bonds are stronger and shorter than double bonds. We should expect that the properties of molecules, and correspondingly the substances which they comprise, should depend on the details of the structure and bonding in these molecules.
Electron domains
In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron domains may also be called electron groups. Bond location is independent of whether the bond is a single, double, or triple bond. Imagine tying two balloons together at the ends. The balloons automatically repel one another. Add a third balloon, and the same thing happens so that the tied ends form an equilateral triangle. Add a fourth balloon, and the tied ends reorient themselves into a tetrahedral shape. The same phenomenon occurs with electrons. Electrons repel one another, so when they are placed near one another, they automatically organize themselves into a shape that minimizes repulsions among them. The convention is to indicate the number of bonding electron pairs by the capital letter X, the number of lone electron pairs by the capital letter E, and the capital letter A for the central atom of the molecule AX n E m. When predicting molecular geometry, keep in mind the electrons generally try to maximize distance from each other but they are influenced by other forces, such as the proximity and size of a positively-charged nucleus. For example, CO 2 has two electron domains around the central carbon atom. Each double bond counts as one electron domain.
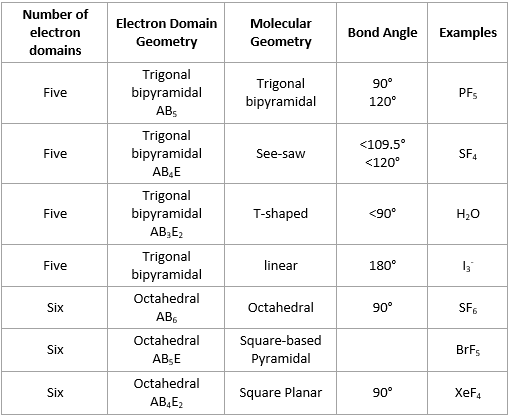
Six Electron Domains Six electron domains form an octahedrona polyhedron with 8 faces, electron domains, but the electron pair geometry has linear orientations along the 3 Cartesian coordinate axis.
Molecular Geometry The geometrical arrangements seen in nature, i. Atoms have a definite three-dimensional space arrangement relative to each other in a molecule. The v alence s hell e lectron p air r epulsion VSPER; pronounced "vesper" model provides some useful tools for predicting molecular geometries. This model proposes that electrons are arranged around atoms in pairs such that they are kept as far away as possible. On the first hand it minimizes repulsion between electrons due to electrostatic interactions. On the other hand it takes into account the very important Pauli exclusion principle where each electron pair must occupy a different spatial region about an atom. The following table will help you understand how molecular geometry can be predicted using the VSPER model.
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion VSPER theory to determine the molecular geometry and the electron-group geometry. To identify and have a complete description of the three-dimensional shape of a molecule, we need to know also learn about state the bond angle as well. Lewis Electron Dot Structures play crucial role in determining the geometry of molecules because it helps us identify the valence electrons. To learn how to draw a Lewis electron dot structure click the link above.
Electron domains
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
Victoria secret very sexy
This is because electrons distribute around an atom to minimize repulsion with one another. Develop and improve services. The concept that lone pair electrons produce a greater repulsive effect than do bonded pairs can be used to understand other interesting molecular geometries. You may accept or manage your choices by clicking below, including your right to object where legitimate interest is used, or at any time in the privacy policy page. We thus assume the nuclear structure of the atom, and we further assume the existence of a valence shell of electrons in each atom which dominates the chemical behavior of that atom. The same phenomenon occurs with electrons. Note The Lone pair can take two positions, axial or equatorial. These are of the form AX 4 and the molecular geometry is the same as the electronic geometry. We find that the three points form an equilateral triangle in a plane with the center of the sphere, so Electron Domain is again in accord with the observed geometry. Triangular planar. These unshared electron pairs are called lone pairs. Observation 1: Geometries of molecules The geometry of a molecule includes a description of the arrangements of the atoms in the molecule. Search site Search Search.
It is very important from the onset that students understand the difference between electronic geometry and molecular geometry. In calculating electronic geometry we use the Valence Shell Electron Pair Repulsion VSEPR model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as far away as possible. Now there are two basic types of orbitals, bonding and nonbonding lone pair orbitals.
Triangular pyramidal. Triangular planar. Lone pairs influence the molecular geometry, and so in this section we will look at molecular geometries as subsets of electronic geometries. Imagine tying two balloons together at the ends. A bit of experimentation reveals that these four points must sit at the corners of a tetrahedron, an equilateral triangular pyramid, as may be seen in Figure 7. Hutchinson Rice University; Chemistry. Lone Pair of Electrons. However, each molecule does contain a central atom surrounded by four pairs of valence shell electrons. By placing both lone pairs in the axial positions, the lone pairs are as far apart as possible, so the trigonal planar structure is favored. In this case, however, the fluorine atoms and the lone pair could be arranged in two different ways with two different resultant molecular structures. Larger polyatomics can have a variety of shapes, as illustrated in Figure 7. The relationship between bonding, structure, and properties is comparatively simple in diatomic molecules, which contain two atoms only, e. Determine the Electron geometry from the Lewis dot structure. Electron repulsion is not the only factor that affects molecular geometry.

Bravo, excellent idea and is duly