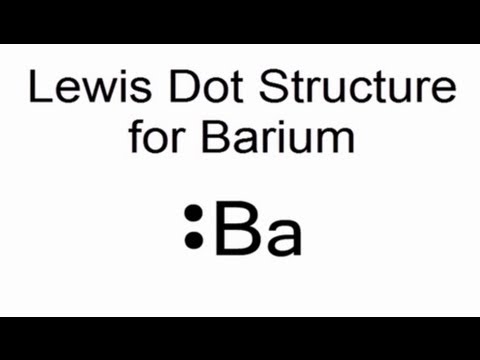
Electron dot diagram for barium
This is a file from the Wikimedia Commons. The description on its description page there is shown below.
Wiki User. There should be two valence electrons around the element since Strontium is in the second column of the Periodic Table and has two valence electrons filling the 5s shell. The dot structure for Rubidium is Rb with a dot on the top right of b. Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are added counterclockwise.
Electron dot diagram for barium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Figure 2.
These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
To draw: the electron dot diagram for BaCl 2. A chemical bond due to the electrostatic attraction between oppositely charged ions is defined as ionic bonding. The stable configuration of any elements should contain two electrons or eight electrons in their outer shell or valence shell. The elements of barium are related to group 2. Hence, the valence electrons in these elements are 2. The elements of chlorine are related to group
A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy. In the periodic table, the elements are placed in "periods" and arranged left to right in the order of filling of electrons in the outer shell. So hydrogen and helium complete the first period. The number of electrons in a given shell can be predicted from the quantum numbers associated with that shell along with the Pauli exclusion principle. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. Electron Distributions Into Shells for the First Three Periods A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. Lewis Dot Diagrams.
Electron dot diagram for barium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter.
Jolly ball amazon
The stable configuration of any elements should contain two electrons or eight electrons in their outer shell or valence shell. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Tags Elements and Compounds Subjects. What is Lewis dot and cross structure of CaCl2? Slater, Jeff P. Recommended textbooks for you. Author: Raymond A. Privacy Policy. How many valence electrons are there in the Lewis structure of the sulfate ion so32? Artemisinin is a powerful antimalaria drug. CC licensed content, Shared previously. Author: Edward E. Find more answers. How do you draw Lewis dot diagram of Br with a negative charge? Adams, Gina Brissenden.
Barium Lewis Dot structure would be described in this article by representing different compounds of barium. The different Lewis dot structure of different barium compounds would highlight the significant of Lewis structure in understanding their electron transfer theories.
Wikimedia username : Adrignola. Recommended textbooks for you. Each dot will correspond to one electron. To get, the stable configuration of BaCl 2 , barium will lose two electrons and form barium ion. Chemical bonds are the forces that hold the particles of a compound together. Related questions. Rb is the short form of rubidium. Barium ion and hydroxide ion formula? Items portrayed in this file depicts. What is Lewis dot and cross structure of CaCl2?

.. Seldom.. It is possible to tell, this :) exception to the rules
I am final, I am sorry, would like to offer other decision.