Electron withdrawing groups list
Open navigation menu. Close suggestions Search Search. User Settings.
Homework problems? Exam preparation? Trying to grasp a concept or just brushing up the basics? Our proven video lessons ease you through problems quickly, and you get tonnes of friendly practice on questions that trip students up on tests and finals. Our personalized learning platform enables you to instantly find the exact walkthrough to your specific type of question. Activate unlimited help now! You can still navigate around the site and check out our free content, but some functionality, such as sign up, will not work.
Electron withdrawing groups list
Hey there! We receieved your request. Electron withdrawing groups through resonance effect:. Electron donating groups through resonance effect:. Please choose valid name. Please Enter valid email. Please Enter valid Mobile. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade. Register Now. We receieved your request Stay Tuned as we are going to contact you within 1 Hour.
Hey there! Otherwise: [20] The most-activating substituent usually controls over the less-activating one.
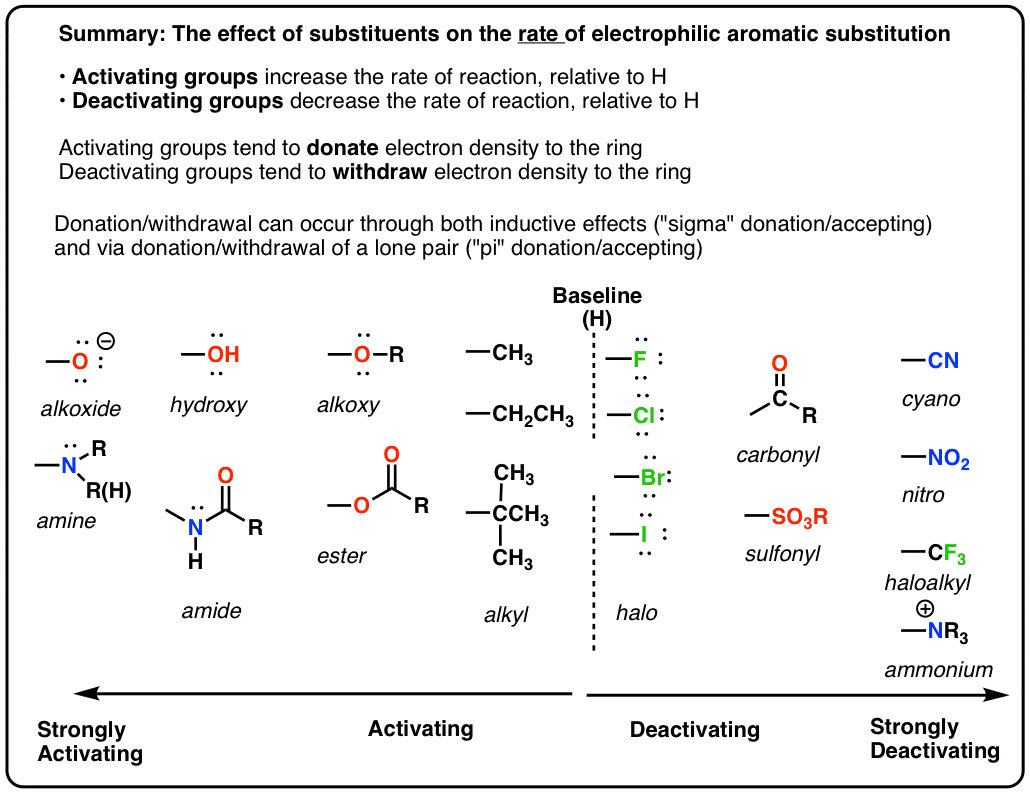
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. EDGs are therefore often known as activating groups , though steric effects can interfere with the reaction. An electron withdrawing group EWG will have the opposite effect on the nucleophilicity of the ring. EDGs and EWGs also determine the positions relative to themselves on the aromatic ring where substitution reactions are most likely to take place. Electron donating groups are typically divided into three levels of activating ability The "extreme" category can be seen as "strong". Electron withdrawing groups are assigned to similar groupings.
Electrophilic Aromatic Substitution: Introduction. Electrophilic Aromatic Substitution — The Mechanism. Last post in this series we introduced electrophilic aromatic substitution. Why is this a substitution reaction, you ask? But not yet. Sure, you can make guesses — even good ones! But the ultimate test of a mechanistic hypothesis is how well it fits with experiment, and that typically involves a lot of lab work.
Electron withdrawing groups list
Although the calculations described in this section will help you understand the principles of NMR, it is the actual delta values, not the calculations, which are of greatest importance to the beginning organic chemist. Thus, we shall try to focus on the interpretation of NMR spectra, not the mathematical aspects of the technique. In Section Although you will eventually be expected to associate the approximate region of a 1 H NMR spectrum with a particular type of proton, you are expected to use a general table of 1 H NMR chemical shifts such as the one shown in Section
Fernando cruz tattoo
Please check your email for login details. Culture Documents. Syed Arsalan Syed Arsalan. London: Cambridge University Press. Organic chemistry : structure and function. There is no resonance effect because there are no orbitals or electron pairs which can overlap with those of the ring. Flag for inappropriate content. ISSN Ciclos de Born Ciclos de Born. Substituents add ortho to the amine in diethyl- meta -trifluoromethyl aniline and ortho to the fluoride in para -fluorobenzaldehyde When multiple substituents are comparably activating, steric hindrance dominates regioselectivity. Substituents add ortho to the methyl group in para - tert -butyl toluene In particular, the position between two substituents, each meta to the other, reacts last. ISBN However, the dimer form is less stable in a solution. Cancel Notify me.
The above reaction would more readily proceed if the electrophilicity of the carbonyl carbon were enhanced.
Last Viewed. Nitrogen has a lone pair of electrons. Article Talk. Nabbs; Alan, R. We track the progress you've made on a topic so you know what you've done. London: Cambridge University Press. Syed Arsalan Syed Arsalan. The Hammond postulate then dictates that the relative transition state energies will reflect the differences in the ground state energies of the Wheland intermediates. Download as PDF Printable version. For example, aniline has resonance structures with negative charges around the ring system: The amino group can donate electron density through resonance.

I consider, that you are not right. I am assured. Write to me in PM, we will talk.
I consider, that you are mistaken. Let's discuss it. Write to me in PM.
Also what from this follows?