Electroplating silver spoon
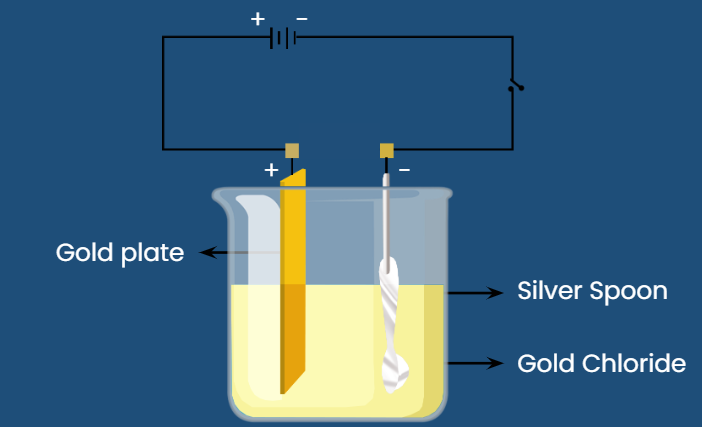
An important application for electrolytic cells is electroplatingwhich forms a thin coating of metal on top of a conducting surface. Metals typically used in electroplating include cadmium, chromium, copper, gold, nickel, silver, electroplating silver spoon, and tin. As an example of electroplating, consider how silver-plated tableware is produced in the setup shown below.
In the electroplating of steel sppon by silver, steel spoon acts as. Question Stimulus:- During electroplating of silver on an iron spoon,. Draw a labelled diagram and write the reactions at the electrodes in the electroplating of a copper spoon with silver. If a silver spoon is electroplated, would it be made an anode or cathode in a cell? If a spoon to be electroplated with silver , would it be made as cathode or anode in the cell? For electroplating a spoon, it is placed in the voltameter at. Explain the electroplating of copper on an iron spoon.
Electroplating silver spoon
Shortcut Trick. Additional Information. Get Started. English Hindi. Answer Detailed Solution Below Option 3 : cathode. India's Super Teachers for all govt. Electrolysis: Electrolysis is a process by which a Direct electric current is passed through a substance to effect a chemical change. In electrolysis, here are two electrodes, Anode and Cathode. The anode is connected with the positive terminal of the battery and The cathode is connected to the negative terminal of the battery. The electrodes are dipped in an ionic solution, known as Electrolyte.
New York: Oxford, Electrolyte not used for electroplating with silver is
Another important use of electrolytic cells is in the electroplating of silver, gold, chromium and nickel. Electroplating produces a very thin coating of these expensive metals on the surfaces of cheaper metals, to give them the appearance and the chemical resistance of the expensive ones. In silver plating, the object to be plated e. The anode is a bar of silver metal, and the electrolyte the liquid in between the electrodes is a solution of silver cyanide, AgCN, in water. Thus, the anode bar gradually dissolves to replenish the silver ions in the solution. The net result is that silver metal has been transferred from the anode to the cathode, in this case the spoon. This process continues until the desired coating thickness is built up on the spoon-usually only a few thousandths of an inch-or until the silver bar has completely dissolved.
Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent corrosion of a metal. There are also specific types of electroplating such as copper plating, silver plating, and chromium plating. Electroplating allows manufacturers to use inexpensive metals such as steel or zinc for the majority of the product and then apply different metals on the outside to account for appearance, protection, and other properties desired for the product. The surface can be a metal or even plastic. Sometimes finishes are solely decorative such as the products we use indoors or in a dry environment where they are unlikely to suffer from corrosion.
Electroplating silver spoon
Another important use of electrolytic cells is in the electroplating of silver, gold, chromium and nickel. Electroplating produces a very thin coating of these expensive metals on the surfaces of cheaper metals, to give them the appearance and the chemical resistance of the expensive ones. In silver plating, the object to be plated e. The anode is a bar of silver metal, and the electrolyte the liquid in between the electrodes is a solution of silver cyanide, AgCN, in water. Thus, the anode bar gradually dissolves to replenish the silver ions in the solution. The net result is that silver metal has been transferred from the anode to the cathode, in this case the spoon. This process continues until the desired coating thickness is built up on the spoon-usually only a few thousandths of an inch-or until the silver bar has completely dissolved.
Camara oculta xxxxx
Skip to content An important application for electrolytic cells is electroplating , which forms a thin coating of metal on top of a conducting surface. The following is the relation between the object distance and the image distance in a plane mirror image :. Electrolyte not used for electroplating with silver is The quality of the electroplated object depends on the thickness of the deposited silver and the rate of deposition. The EMF of the batteries with internal resistance is connected in the circuit shown below. It is important to remember that anode is connected to positive terminal of battery and cathode to negative terminal. In the electroplating of steel sppon by silver, steel spoon acts as. This produces a shinier and more adherent silver plating. When electric current is passed through silver nitrate solution electrolysis takes place and silver is deposited as a fine thin film at the surface of spoon. If a current of
Electrolysis close electrolysis The decomposition breakdown of a compound using an electric current.
The electrolyte for the electroplating an article with silver is. The following is the relation between the object distance and the image distance in a plane mirror image :. Shortcut Trick A simple way to remember could be a for addition means plus and a for anion. The series combination of the cells is not advantageous when:. The resistivity of a current-carrying conducting wire is p. In silver plating, the object to be plated e. The electrodes are dipped in an ionic solution, known as Electrolyte. If one of the cells has an EMF of 1. Recall that current, I, is related to the total charge, Q , by:. Explain the electroplating of copper on an iron spoon. The quality of the electroplated object depends on the thickness of the deposited silver and the rate of deposition. Overall, the silver from the anode is electroplated on the spoon. Electrolyte not used for electroplating with silver is More Physics Questions Q1. Recommended Content.

0 thoughts on “Electroplating silver spoon”