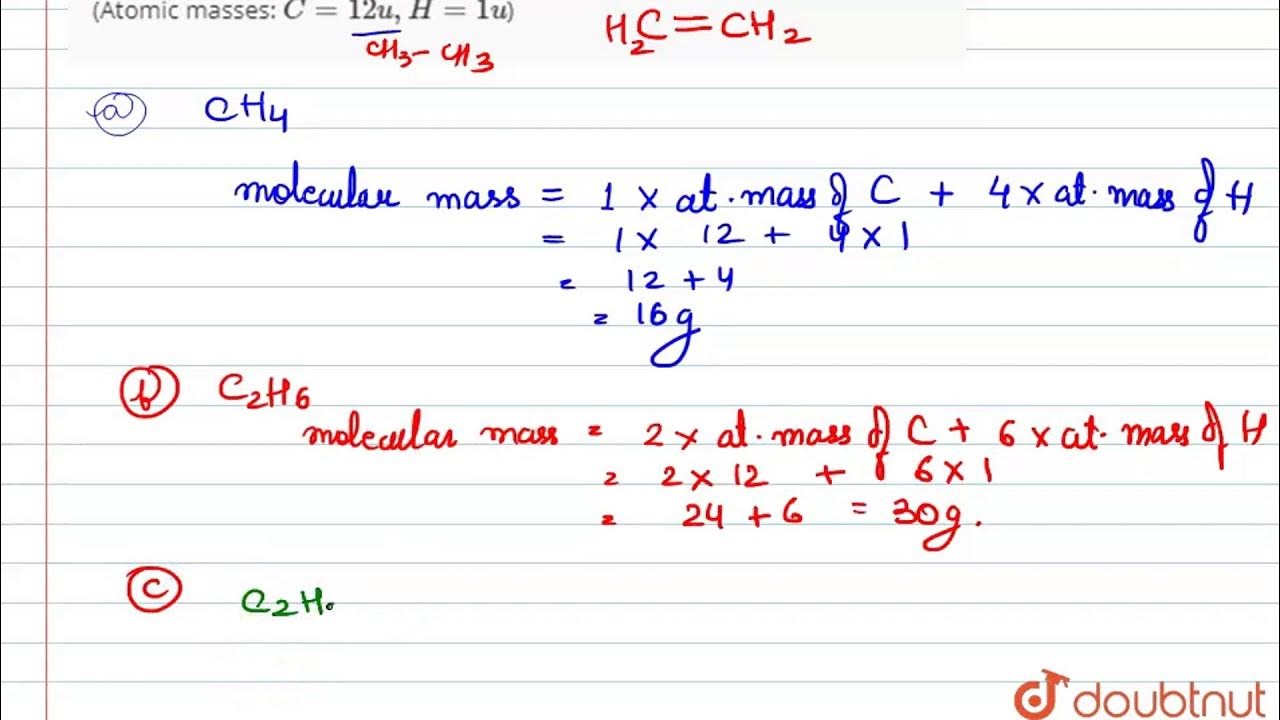
Ethane molecular mass
Additional Information.
The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro.
Ethane molecular mass
Molar mass of Ethane C 2 H 6 is Then, lookup atomic weights for each element in periodic table : C: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in C 2 H 6 : C: 2, H: 6 Then, lookup atomic weights for each element in periodic table : C: Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa.
Precautionary statements. Categories : Ethane Alkanes Industrial gases Greenhouse gases. For example, an ethyl group linked to a hydroxyl group yields ethanolthe alcohol in beverages.
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group ; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol , the alcohol in beverages. Ethane was first synthesised in by Michael Faraday , applying electrolysis of a potassium acetate solution.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Ethane molecular mass
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Lawlor safety
In which category of carcinogens radio frequency of mobiles phones is kept? Ethane can also be separated from petroleum gas , a mixture of gaseous hydrocarbons produced as a byproduct of petroleum refining. April 26, Prior to the s, ethane and larger molecules were typically not separated from the methane component of natural gas, but simply burnt along with the methane as a fuel. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Rotating a molecular substructure about a twistable bond usually requires energy. The disease caused by breathing polluted air is:. EC Number. Ethane can react with the halogens , especially chlorine and bromine , by free-radical halogenation. Y verify what is Y N? Which of the following is NOT an organ?
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining.
How many hydrogen atoms are there in a molecule of methane? Autoignition temperature. One mole contains exactly 6. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Higher alkanes List of alkanes. Privacy Policy We don't collect information from our users. Bibcode : GeoRL.. SbH 3. Ethane occurs as a trace gas in the Earth's atmosphere , currently having a concentration at sea level of 0. Gas laws. University of Michigan. Sherwood; Blake, Donald R. R—X is the general formula of which functional group in which one or more hydrogen atoms are replaced by Group 17 elements?

0 thoughts on “Ethane molecular mass”