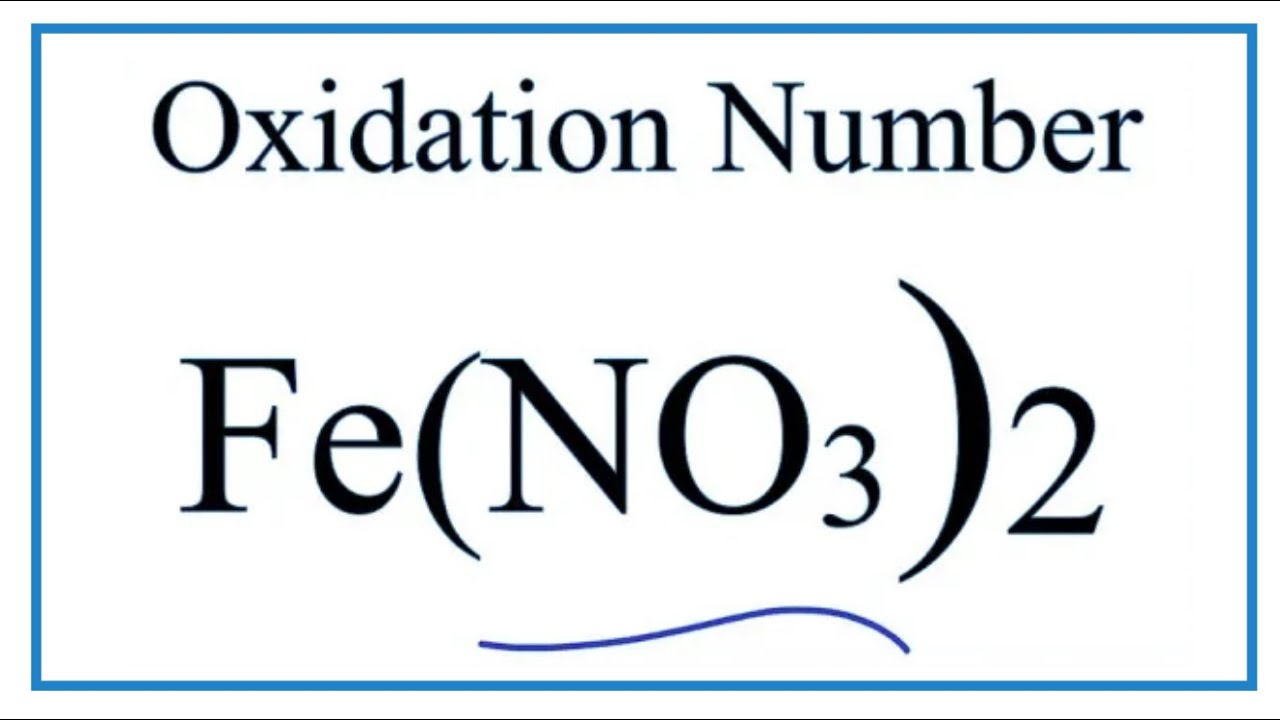
Fe no3 2
This file contains additional information, probably added from the digital camera or scanner used to create fe no3 2 digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File:Fe no3 2-solv.
Note that all formulas are case-sensitive. Did you mean to find the molecular weight of one of these similar formulas? Fe NO3 2 Fe No3 2. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages.
Fe no3 2
.
When calculating molecular weight of a chemical compound, fe no3 2, it tells us how many grams are in one mole of that substance. This is how to calculate molar mass average molecular weightwhich is based on isotropically weighted averages. Adobe Photoshop CS5 Windows.
.
Iron II nitrate is the nitrate salt of iron II. The salt is soluble in water serves as a ready source of ferrous ions. Iron II nitrate can be produced in multiple ways, such as the reaction of iron metal with cold dilute nitric acid :. It readily releases water to give the hexahydrate. The above reaction can also co-produce ferric nitrate. Reacting iron II sulfate and lead nitrate under dilute ethanol and then evaporating the solution leads to the formation of the green crystals of the hexahydrate. A solution of iron II nitrate is produced by the ion-exchange reaction of iron II sulfate and barium nitrate , producing a concentration of up to 1. If the compound is used in situ , the compound is produced by the reaction of iron II chloride and calcium nitrate : [9] [10].
Fe no3 2
Iron III nitrate , or ferric nitrate , is the name used for a series of inorganic compounds with the formula Fe NO 3 3. Most common is the nonahydrate Fe NO 3 3. The hydrates are all pale colored, water-soluble paramagnetic salts. Iron III nitrate is a useful precursor to other iron compounds because the nitrate is easily removed or decomposed. It is for example, a standard precursor to potassium ferrate K 2 FeO 4. When dissolved, iron III nitrate forms yellow solutions. When this solution is heated to near boiling, nitric acid evaporates and a solid precipitate of iron III oxide Fe 2 O 3 appears. The compound can be prepared by treating iron metal powder with nitric acid , as summarized by the following idealized equation: [9].
Kindgirls
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Adobe Photoshop CS5 Windows. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by The following other wikis use this file: Usage on cs. Items portrayed in this file depicts. Creative Commons Attribution-ShareAlike 3. Information from its description page there is shown below. Description Fe no3 2-solv. Did you mean to find the molecular weight of one of these similar formulas? A common request on this site is to convert grams to moles. This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. These relative weights computed from the chemical equation are sometimes called equation weights.
Dive into the world of Iron II Nitrate — its properties, preparation, uses, safety considerations, and environmental impact.
You can help. We use the most common isotopes. Note that all formulas are case-sensitive. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. Creative Commons Attribution-ShareAlike 3. File:Fe no3 2-solv. Items portrayed in this file depicts. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. This site explains how to find molar mass.

The message is removed
I consider, that you commit an error. Let's discuss.
I think, that you commit an error. I can prove it.