Fe2o3 fe so4 3
The equilibrium composition of the reference gas at the measuring temperatures was computed using the thermodynamic data on the gaseous species reported in the literature. A mixture of ferric oxide and sulfate was kept in a closed system to ensure establishment of equilibrium partial pressure at the electrode, fe2o3 fe so4 3. Uncertainties arising from the formation of sulfate solid solution were thus eliminated. There is no evidence for the formation of oxysulfates in fe2o3 fe so4 3 Fe-S-0 system.
An iron sulfate of nominal composition Fe 2 SO 4 3 H 2 O 5 has been synthesized and its structure determined and refined by high resolution powder diffraction using synchrotron radiation. Weight loss experiments show that it is currently a pentahydrate, despite earlier reports that lausenite is a hexahydrate. We argue that our synthetic material provides a structure determination for the type specimen of lausenite. Your purchase has been completed. Your documents are now available to view. From the journal American Mineralogist.
Fe2o3 fe so4 3
International Hazard. National Hazard. Hazard to Others. Super Administrator. The art of wondering makes life worth living Want to wonder? Not logged in [ Login ]. Back to:. Printable Version. I'm having troubles producing iron sulfate I must start with ferric oxide and use sulfuric acid to produce iron sulfate but ive been having some problems
Accepted: American Mineralogist, Vol. Since sodium oxide is neutralized by the addition of sulphuric acid, the ash of the catalyst is almost entirely Fe 2 O 3.
Iron III oxides with corundum , bixbyite , spinel and orthorhombic structures were identified as solid products of this conversion. A significant influence of the heating temperature on the decomposition mechanism and on the phase composition of reaction products was found. To see the editorial board, please visit the website of Springer Nature. For subscription options, please visit the website of Springer Nature. Sign in Sign up. Advanced Search Help.
Please enter the reactant or product to start the search. Note: Separate each reactant with a single space, e. Reaction conditions when applied Fe 2 SO 4 3. Reaction process Fe 2 SO 4 3. The result of the reaction Fe 2 SO 4 3.
Fe2o3 fe so4 3
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables.
Score of the baltimore orioles baseball game
Although sulphuric acid is currently used as the standard catalyst in numerous industrial processes, the catalytic activity per unit mass of the carbon-based catalyst rivals that of sulphuric acid in many reactions. A highly active, carbon-based solid acid catalyst has been reported 1 , 2 , 3. Quote: Originally posted by MMendoza Ethics declarations Competing interests The authors declare no competing financial interests. Our Blog Akademiai. Download options Please wait Download references. Anhydrous iron sulphate: an example of weak ferrimagnetism. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. Toda, M. Chemical composition of tomato seeds affected by conventional and organic production systems.
.
Unit converters. Thermodynamic properties of the Tschermak solid solution in Fe-chlorite: Application to natural examples and possible role of oxidation. Ingraham: Can. What form is it in - granules, powder etc.? Downloaded on Journal of Radioanalytical and Nuclear Chemistry, — a citation-based bibliography and impact analysis using Hirsch-type statistics. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. If MCNC-SA is to be prepared with sufficient magnetic properties that it may be magnetically separated from the reaction solution, the lower limit for the concentration of the iron nitrate solution applied during synthesis appears to be 1. Knutsen and A. Authors: F. Use a bit more concentrated sulfuric acid, 1M acid is not even good for washing my hands if it get's dirty in the lab Article Google Scholar Liu, X. Show results from All journals This journal. Search articles by author W. This method uses algebraic equations to find the correct coefficients.

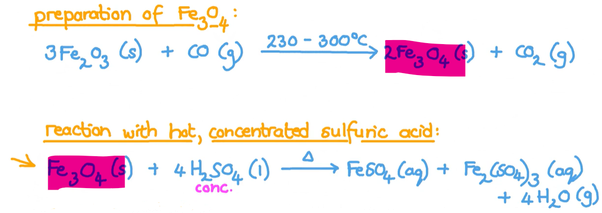
0 thoughts on “Fe2o3 fe so4 3”