Fenbendazole dosage for humans
When fenbendazole became difficult to obtain on the market, even the human anthelmintic albendazole was sold out.
Fenbendazole is a benzimidazole-class anthelmintic that is used for the control of immature and adult stages of internal parasites, such as nematodes and trematodes, in domestic food-animal species. It is not approved by the United States Food and Drug Administration for treating pheasants despite Syngamus trachea being one of the most prevalent nematodes that parasitize pheasants. Because it is a highly effective treatment, e. Therefore, we conducted a risk assessment to evaluate the potential repeat-dose and reproductive, teratogenic, and carcinogenic human risks that may be associated with the consumption of tissues from pheasants that were previously treated with fenbendazole. We conducted a quantitative risk assessment applying both deterministic and stochastic approaches using different fenbendazole sulfone residue limits tolerance, maximum residue limits, and analytical limit of detection established in different poultry species by the Food and Drug Administration, the European Medicines Agency, and other regulatory agencies in Japan, Turkey, and New Zealand. Our results show that fenbendazole poses minimal risk to humans when administered to pheasants in an extra-label manner, and a comparison of different fenbendazole sulfone residue limits can help assess how conservative the withdrawal interval should be after extra-label drug use.
Fenbendazole dosage for humans
Case Rep Oncol 1 September ; 14 2 : — Fenbendazole is a benzimidazole anthelmintic agent, with a broad antiparasitic range in animals such as dogs and pigs. The agent is also reported to exert antitumor effects and inhibit microtubule-associated tubulin polymerization, but its safety and tolerability profile in humans remains unclear. An year-old female patient with advanced nonsmall cell lung cancer NSCLC was started on pembrolizumab monotherapy. The patient experienced severe liver injury 9 months later. An interview with her and her family revealed that she had been taking fenbendazole for a month, solely based on social media reports suggesting its effectiveness against cancer. The antitumor inhibitory effects of fenbendazole have been reported; however, she did not experience tumor shrinkage. This is the first case report of a patient with advanced NSCLC who self-administered the anthelmintic, fenbendazole. Twitter and Facebook are online social media platforms which have been constructively used to exchange information among cancer patients. However, sources of medical information on these platforms are often unproven, and it is difficult for nonmedical professionals to accurately select and filter complex medical information. Physicians should enquire patients about self-administration of orally ingested products, including dietary supplements, herbs, or bioactive compounds, in cases of unexpected adverse reactions. Fenbendazole methyl N - 6-phenylsulfanyl-1 H -benzimidazolyl carbamate is a benzimidazole compound with broad antiparasitic spectrum use in various animals [1].
Fenbendazole also seems to have higher efficacy when administered over several days Even though the Korean Ministry of Food and Drug Safety warned that this claim was unfounded and that side effects of such drugs could damage patients, the outbreak of the anthelmintics is still ongoing. Food US.
Each gram of paste contains milligrams mg fenbendazole 10 percent. See No. A For the treatment and control of large strongyles Strongylus edentatus, S. B For treatment and control of ascarids Parascaris equorum. D For the control of arteritis caused by fourth-stage larvae of Strongylus vulgaris in horses. Do not use in horses intended for human consumption. Administer orally 2.
Take the required amount of fenbendazole after a high fat meal daily for 6 days, skip on the seventh day and repeat weekly. Fenbendazole mg. Take three times a week, once a day after a high fat meal. Then take no fenbendazole for four days. Repeat the cycle every week. Repeat for 10 weeks. Then Stop for 10 weeks. Repeat the cycle. One study published in by Dr.
Fenbendazole dosage for humans
It belongs to a family of drugs called benzimidazoles, which have been safely used around the world as anthelmintics deworming medications for animals for well over half a century [1]. Fenbendazole is commonly used in veterinary medicine for the treatment of gastrointestinal parasites like giardia, roundworms, hookworms, whipworms, and pinworms [2]. A sister product to fenbendazole, called mebendazole, is typically found in deworming medications for humans [1]. In recent decades, it has come to light that fenbendazole and other medications of the same family exhibit potent anticancer effects in laboratory in vitro and animal in vivo studies [2]. Benzimidazole anthelmintics such as fenbendazole, albendazole, and mebendazole have been shown to be toxic to cancer cells and minimally toxic to normal cells, induce apoptosis programmed cell death and autophagy cellular repair and regeneration , impair glucose uptake of cancer cells, prevent angiogenesis blood vessel formation of tumors , inhibit drug resistance, and exhibit other antitumor effects in preclinical studies with early clinical research ongoing to observe safety and efficacy in humans [3] [4] [5].
Past life regression sydney
Thus, a tornado graph was created, and correlation coefficients applying Spearman rank were calculated to carry out the sensitivity analysis. Supplemental new animal drug application. J Anim Plant Sci. Their findings in toxicological studies in mice, rats, rabbits, and dogs demonstrated some no-observed-adverse-effect levels NOAELs for different toxicities. We evaluated multiple scenarios using both deterministic and stochastic approaches. Here, we report the case of a patient with advanced nonsmall cell lung cancer NSCLC who obtained information on the antitumor activity of fenbendazole via social media. Therefore, when carrying out the calculations with the pheasant LOD distribution, which has the smallest most likely value 0. Pharmacokinetics, safety, and tolerability of oxfendazole in healthy volunteers: a randomized, placebo-controlled first-in-human single-dose escalation study. D For the control of arteritis caused by fourth-stage larvae of Strongylus vulgaris in horses. World Animal Health Organization O. However, commercial pheasants are a relatively small commodity group compared with commercial chickens and turkeys. Therefore, we conducted a risk assessment to evaluate the potential repeat-dose and reproductive, teratogenic, and carcinogenic human risks that may be associated with the consumption of tissues from pheasants that were previously treated with fenbendazole. Because it is a highly effective treatment, e. We stopped fenbendazole administration immediately, and her liver function parameters gradually improved.
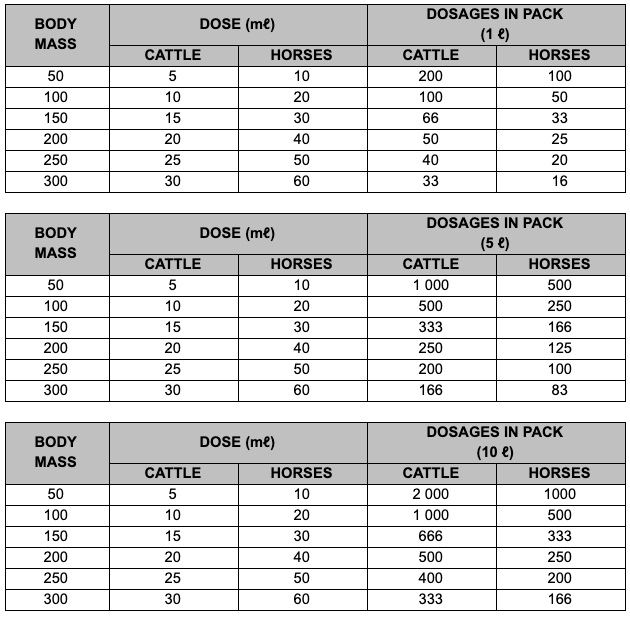
We get many questions on Fenbendazole dosage.
Shimizu, and Y. Page Views BMC Public Health. Plasma pharmacokinetics of midazolam in chickens, turkeys, pheasants and bobwhite quail. Anti-nematodal drugs. The possibility of liver injury as an immune-related adverse event was also considered, since our patient was also using pembrolizumab. Published by S. Fenbendazole also seems to have higher efficacy when administered over several days D For the control of arteritis caused by fourth-stage larvae of Strongylus vulgaris in horses. Treatment of nematodiasis in poultry and game birds with fenbendazole. The human food consumption risk assessment was developed and implemented using Risk version 7. Disclosure: The author has no potential conflicts of interest to disclose. Also, the EMA did not observe any carcinogenetic effects regardless of the dose of fenbendazole used. Cytochrome Pdependent metabolism of midazolam in hepatic microsomes from chickens, turkeys, pheasant and bobwhite quail.

Here and so too happens:)
I can suggest to come on a site where there are many articles on a theme interesting you.