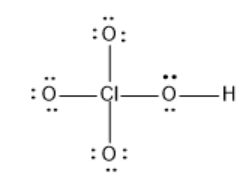
Formal charge of cl
The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We calculate the formal charge formal charge of cl an atom in a molecule or polyatomic ions as follows:, formal charge of cl.
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure.
Formal charge of cl
.
Solution Assign one of the electrons in each Br—Cl bond to the Br atom and one to the Cl atom in that bond: Assign the lone pairs to their atom.
.
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. But first, let's introduce a concept we will refer back to frequently for the rest of this term: electronegativity. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons or electron density towards itself. It determines how the shared electrons are distributed between the two atoms in a bond. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity.
Formal charge of cl
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion. We must remember that the formal charge calculated for an atom is not the actual charge of the atom in the molecule.
Asajj ventress sith
CC licensed content, Shared previously. Solution Assign one of the electrons in each Br—Cl bond to the Br atom and one to the Cl atom in that bond: Assign the lone pairs to their atom. The structure with a terminal oxygen atom best satisfies the criteria for the most stable distribution of formal charge:. The skeleton structures of these species are shown: Write the Lewis structures for the following, and include resonance structures where appropriate. The structure that gives zero formal charges is consistent with the actual structure:. A few guidelines involving formal charge can be helpful in deciding which of the possible structures is most likely for a particular molecule or ion:. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion. Just as a rhinoceros is neither a dragon sometimes nor a unicorn at other times, a resonance hybrid is neither of its resonance forms at any given time. Search site Search Search. The structure with formal charges of 0 is the most stable and would therefore be the correct arrangement of atoms.
Skip to main content. Table of contents.
As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. Assign one of the electrons in each Br—Cl bond to the Br atom and one to the Cl atom in that bond:. As another example, the thiocyanate ion, an ion formed from a carbon atom, a nitrogen atom, and a sulfur atom, could have three different molecular structures: CNS — , NCS — , or CSN —. Indicate which of the three has the strongest carbon-oxygen bond. Sodium nitrite, which has been used to preserve bacon and other meats, is an ionic compound. One oxygen atom must have a double bond to carbon to complete the octet on the central atom. Subtract this number from the number of valence electrons for the neutral atom. The structure that gives zero formal charges is consistent with the actual structure:. Again, experiments show that all three C—O bonds are exactly the same. Is the actual structure consistent with the formal charges?

0 thoughts on “Formal charge of cl”