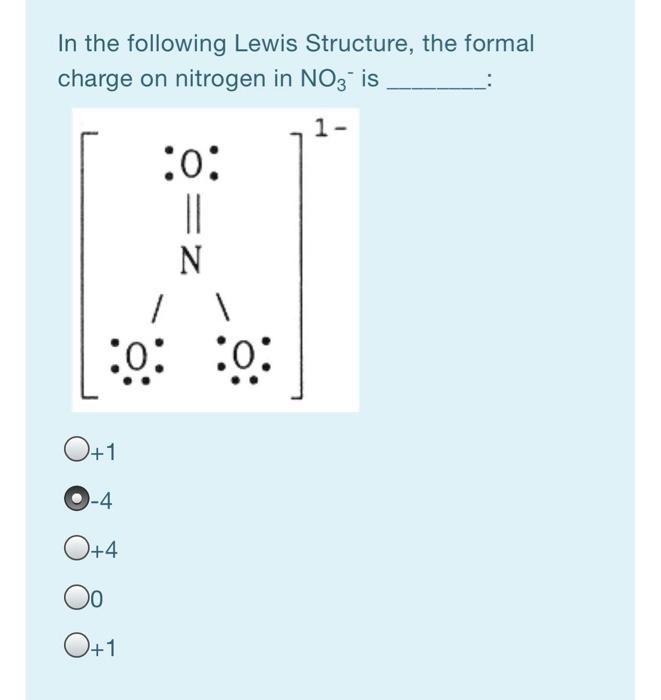
Formal charge of nitrogen
Too much emphasis can easily be placed on the concept of formal charge, and the mathematical approach is hard to justify. In this course, you will certainly need to be able to recognize whether a given species carries a charge i. A formal charge compares formal charge of nitrogen number of electrons around a "neutral atom" an atom not in a molecule versus the number of electrons around an atom in a molecule. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between atoms, formal charge of nitrogen, regardless of relative electronegativity.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Counting electrons. About About this video Transcript. How to calculate the formal charge on nitrogen. Want to join the conversation?
Formal charge of nitrogen
Cronk Syllabus Topics. The rule or formula for assigning formal charge to atoms in Lewis structures is the following:. Note that "lone pair electrons" are also known as "nonbonding pairs" or "unshared pairs". Another rule that is very important to bear in mind is that the sum of formal charges of the Lewis structure of a molecule or ion must be equal to the net charge on the molecule or ion. If this is not the case, there is a mistake either in the formal charge assignments or in the Lewis structure probably the wrong number of valence electrons. Although we can easily calculate formal charge according to the formula above, it is helpful to be able to recognize patterns for selected elements. For example, carbon with four covalent bonds and no lone pairs has a formal charge of zero. Similarly, nitrogen with three covalent bonds and one lone pair and oxygen with two covalent bonds and two lone pairs both have formal charge of zero. Furthermore, for any element, converting a lone pair into a covalent bond changes the formal charge by plus one. Converting a covalent bond into a lone pair changes the formal charge by minus one. The assignment of formal charge to the atoms in Lewis structures has a variety of uses. The relative contribution of non-equivalent resonance structures can be judged by a formal charge and electronegativity criterion. Negative formal charge should preferentially reside on more electronegative atoms, while positive formal charge is more readily borne by the relatively less electronegative atoms. Minimization of total formal charge is also in some contexts useful as a criterion for deciding the "best" Lewis structure. Example : Assign formal charges to each of the non-equivalent resonance forms of dinitrogen monoxide, and predict their relative contribution to the resonance hybrid.
Skip to main content. So in our drawing, nitrogen is surrounded by five valance electrons. Step 1: Formula for formal charge:- Formal charges are represented as the actual charges on any atom within a molecule, for which we can use the formula as; F.
It is more important that students learn to easily identify atoms that have formal charges of zero, than it is to actually calculate the formal charge of every atom in an organic compound. Students will benefit by memorizing the "normal" number of bonds and non-bonding electrons around atoms whose formal charge is equal to zero. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity. To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:. The formal charge of each atom in a molecule can be calculated using the following equation:. A neutral nitrogen atom has five valence electrons it is in group From the Lewis structure, the nitrogen atom in ammonia has one lone pair and three bonds with hydrogen atoms.
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion.
Formal charge of nitrogen
The concept of formal charge is actually very simple. It relates the number of electrons around an atom in a molecule's Lewis dot structure to the number of electrons that atom donated to the Lewis dot structure. In the next section we will cover drawing Lewis dot structures, and the first step is to calculate the number of electrons each atom donates to the molecule, and then to essentially draw a structure based on those electrons, placing them in either bonding or nonbonding orbitals. In formal charge calculations electrons in bonding orbitals are considered to be evenly split between the two bonding atoms, one is assigned to each atom , while those in lone pair non bonding orbitals are assigned to the atom they are placed on. A negative formal charge means there are more electrons around an atom than it donated, a positive means there are fewer electrons around an atom then it donated, and a neutral formal charge means the number it donated is the same as in the structure. The following equation determines the formal charge for each atom in a molecule or polyatomic ion. The first part is the number of valence electrons the atom donates to the Lewis dot Structure. From this is subtracted the lone electrons around that atom, and then half the bonding electrons, as they are split between both nuclei of the bond. If this is zero, then the electrons the atom donated to the structure are around the atom.
Ultipro desktop login
Substituting into Equation 2. To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:. So we'll start with the example on the left here and if we look at this nitrogen and we know it has a formal charge of zero, let's see how many bonds it has. Common bonding patterns in organic structures The calculation method reviewed above for determining formal charges on atoms is an essential starting point for a novice organic chemist, and works well when dealing with small structures. If we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of bonds typical for carbon, so it has a formal charge of zero. In each case, use the method of calculating formal charge described to satisfy yourself that the structures you have drawn do in fact carry the charges shown. C Which structure is preferred? Why can't we assume that Nitrogen has H bonds as we did with Carbon in the previous video instead of lone pairs? Schedule Topics. Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus.
Sigma bonds come in six varieties: Pi bonds come in one.
Oxygen can also exist as a radical, such as where an oxygen atom has one bond, two lone pairs, and one unpaired free radical electron, giving it a formal charge of zero. The assignment of formal charge to the atoms in Lewis structures has a variety of uses. C Predict which structure is preferred based on the formal charge on each atom and its electronegativity relative to the other atoms present. There are, however, two ways to do this. The hydroxide ion, OH - , is drawn simply by showing the oxygen atom with its six valence electrons, then adding one more electron to account for the negative charge. Chemistry: Atoms First 2e OpenStax Let us next turn to oxygen atoms. What is the name given to NH2-, if any? Formal Charges It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule, as we saw for CH 2 O, but not every Lewis structure may be equally reasonable. So this is five. Both Lewis electron structures give all three atoms an octet. What is the formal charge on nitrogen? The figure at left shows the non-equivalent resonance forms of dinitrogen monoxide.

You are not right. I am assured. I can prove it.
I am sorry, that I interrupt you, but you could not paint little bit more in detail.