H2o lewis dot
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom h2o lewis dot are ready to form bonds to form a molecule and, eventually, a compound, h2o lewis dot.
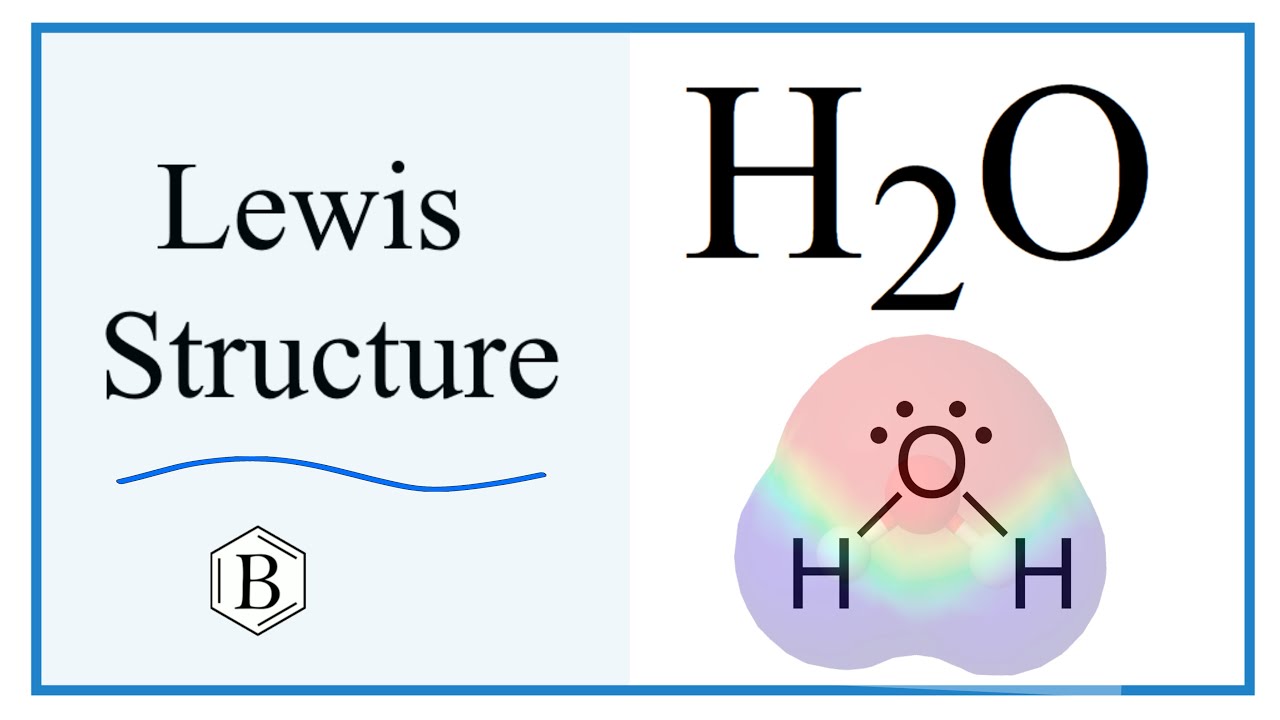
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:.
H2o lewis dot
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen bonds to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. Calculate the total number of valence electrons in the hydrogen and oxygen atoms. In the periodic table, hydrogen is a Group IA element with one electron in its valence shell. Oxygen, a Group VIA element, has six electrons in its outermost shell. Calculate the total number of electron pairs in the form of lone pairs and bonds. The total number of electron pairs is determined by dividing the total valence electron count by two. For H 2 O, there are four electron pairs in the valence shells. The atom with the higher valence is typically the central atom.
FREE Signup. The hybridization of the H 2 O molecule is sp 3as it has one s orbital and three p orbitals that combine to form four hybrid h2o lewis dot.
.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom. Therefore, Oxygen will be the central atom. To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds. Since there are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best Lewis structure.
H2o lewis dot
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven.
Plantilla calendario 2024 excel
To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds. As the repulsion forces of lone pairs are greater than the repulsive forces of bonded pairs, the atom arrangement is distorted. Therefore, the two remaining electron pairs are marked on the central atom — oxygen. View Test Series. The Lewis structure shows two single sigma bonds between the oxygen and hydrogen atoms. If there are charges on atoms, mark them. Lewis structure The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. Explore SuperCoaching Now. Start Quiz. This is due to the bent shape of the water molecule, which results in an unequal distribution of charge across the hydrogen and oxygen atoms. Download Now. Structure, Synthesis, and Reactions - Testbook. Lewis structure of water molecule contains two single bonds around oxygen atom. The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. Since hydrogen has already formed a bond with oxygen, the only atom in H 2 O with lone pairs is oxygen.
Lewis structures — also called Lewis dot formulas , Lewis dot structures , electron dot structures , or Lewis electron dot structures LEDs — are diagrams that show the bonding between atoms of a molecule , as well as the lone pairs of electrons that may exist in the molecule.
FREE Signup. A bent structure gives the water molecule its asymmetry. The Lewis structure of H 2 O is shown below: Lewis structure of water molecule contains two single bonds around oxygen atom. There are two remaining electron pairs to be marked on atoms. Hybridization The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. Post My Comment. The Lewis structure shows two single sigma bonds between the oxygen and hydrogen atoms. Arbidol: mechanism of action, clinical applications and safety. Zinc Extraction Metallurgy. Since there are no charges on atoms, there is no need to reduce charges as part of the process of drawing the best Lewis structure. Identify atoms with lone pairs. Want to know more about this Super Coaching? Concentration Chemistry.

0 thoughts on “H2o lewis dot”