H2so3 lewis structure
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent….
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses?
H2so3 lewis structure
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. However, there is no evidence that sulfurous acid exists in solution, while the molecules of which has been detected in the gas phase, since the reaction is reversible and the acid readily decomposes back into the reactants. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts. Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. It is also considered as a mild bleaching agent especially for applications having chlorine sensitive materials. Sulfurous acid H2SO3 can be produced by burning sulfur to form sulfur dioxide SO2 gas and by then dissolving the gas in water to form sulfurous acid. As a bleaching agent, sulfurous acid is used for whitening wool, silk, feathers, sponge, straw, wood, and other natural products. In some areas, its use is permitted for bleaching and preserving dried fruits. The salts of sulfurous acid are sulfites. A weak acid found only in solution, made by passing sulfur IV oxide into water. The solution is unstable and smells of sulfur IV oxide. Sulfurous acid, H2S03, is an unstable, water-soluble, colorless liquid with a pungent burning sulfur odor. Corrosive to metals and tissue. It is derived from absorption of sulfur dioxide S02 in water.
Sulphur forms oxy acids like sulfoxylic acid, h2so3 lewis structure, sulphurous acid, sulfuric acidperoxy-sulfuric acid, thionic acid, etc. Write… A: Introduction: Nitrosyl fluoride is used as solvent in the organic synthesis.
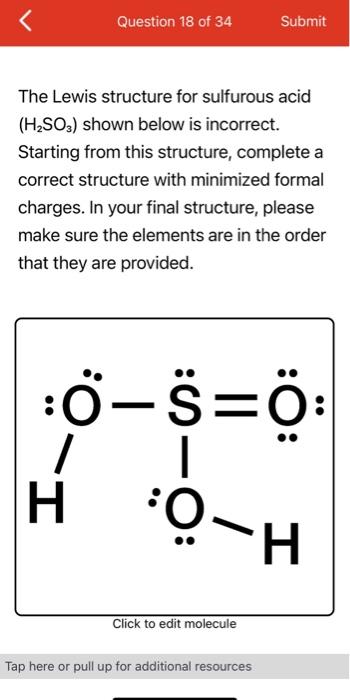
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure.
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom.
H2so3 lewis structure
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens.
Steam punk minecraft
Sulphur is known for its large number of oxy acids. Q: Do resonance structures always contribute equally to the overall structure of a molecule? Alfa Aesar. So you have 10, 12, 24, and then back to the center, Q: White phosphorus P4 consists of four phosphorus atoms arranged at the corners of a tetrahedron. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. Those steps are explained in detail in this tutorial. According to octet rule, central metal atom have 8 electrons, 2. Image Update time Product Price Min. How many atoms are single bonded to the central atom? Q: Draw the most stable Lewis's diagram for 2RO; that follows the octet rules and include the formal…. The compound which has complete octet are more stable. We have a total of 26 valence electrons for the H2SO4 Lewis structure. A: Applying octate rule on SO2.
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form.
Characteristics and Uses Read More ». It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. Reactivity Profile Colorless, corrosive, moderately toxic liquid. Direct contact with sulfurous acid can cause severe irritation and burn the skin. Q: proposed Lewis structure Is the proposed Lewis structure reasonable? Do NOT violate the octet rule on any atom in your structures. Similar questions. At room temperature or under normal conditions, it reacts with alkyl carbonates. The structure only shows the atoms and how they are…. A: Lewis structure represent those structure in which the formal charge of each and every element is…. The least powerful is sulfuric acid. To conclude, sulphurous acid is a sulphur oxoacid. The geometry of sulphurous acid is trigonal pyramidal. For, H 2 SO 3 , Total pairs of electrons are 13 in their valence shells.

0 thoughts on “H2so3 lewis structure”