How many electrons are in chlorine
Examples of Compounds with Chlorine Sodium Chloride This is sodium chloridealso known as table salt. Most people scientist know that the formula for salt is NaCl. One sodium Na atom gives it's electron to one chlorine Cl atom.
Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees. Chlorine Facts and Science. Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. Chlorine has an atomic number of 17 and an atomic mass of As a member of the halogen family on the Periodic Table, chlorine is very reactive with metals and forms salts. The density of chlorine is The high density of chlorine gas causes it to sink if released into the ambient environment.
How many electrons are in chlorine
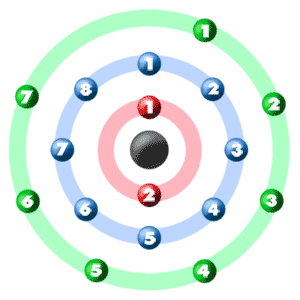
Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in. Chlorine has an atomic number Atomic number is the number of protons present in the nucleus of an atom. All the atoms of an element have same atomic number. In every stable atom the number of electrons is equal to the number of protons. Therefore, the number of electrons is equal to the number of protons in an chlorine atom. This means, that the number of electrons present in an chlorine atom is The electronic configuration of chlorine atom is:- E. Valence electrons are the number of electrons present in the outermost shell of an atom. Now, the last shell of chlorine atom has 7 electrons in it. Therefore, there are 7 valence electrons present in an chlorine atom. Chlorine has seven valence electrons. Chlorine has atomic number The atomic number is the number of protons present in the nucleus of an atom. Stable means that atom has not formed a ion yet.
In the ocean, chlorine is the third most common element, at a prevalence of 1.
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity, behind only oxygen and fluorine. While perhaps best known for its role in providing clean drinking water, chlorine chemistry also helps provide energy-efficient building materials, electronics, fiber optics, solar energy cells, 93 percent of life-saving pharmaceuticals, 86 percent of crop protection compounds, medical plastics, and much more. Elemental chlorine is commercially produced from brine by electrolysis, predominantly in the chlor-alkali process. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus.
How many electrons are in chlorine
Chlorine is a chemical element ; it has symbol Cl and atomic number The second-lightest of the halogens , it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent : among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale , behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval alchemists , which commonly involved the heating of chloride salts like ammonium chloride sal ammoniac and sodium chloride common salt , producing various chemical substances containing chlorine such as hydrogen chloride , mercury II chloride corrosive sublimate , and aqua regia. However, the nature of free chlorine gas as a separate substance was only recognised around by Jan Baptist van Helmont. Carl Wilhelm Scheele wrote a description of chlorine gas in , supposing it to be an oxide of a new element. Because of its great reactivity, all chlorine in the Earth's crust is in the form of ionic chloride compounds, which includes table salt. It is the second-most abundant halogen after fluorine and twenty-first most abundant chemical element in Earth's crust. These crustal deposits are nevertheless dwarfed by the huge reserves of chloride in seawater.
Shiloh jolie-pitt modeling photos
Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too remember, Aluminum has three extra electrons. How many valence electrons are in an atom of magnesium? Therefore, there are 7 valence electrons in an chlorine atom. All of them now have eight electrons, and a filled outer shell! The density of chlorine is Disinfection—Bleach solutions are used extensively in restaurants, schools, hospitals, homes, and other settings to disinfect surfaces, destroying pathogens, including norovirus, hepatitis A, Ebola, influenza, and many more. Paper—Used as an oxidizing and bleaching agent in the pulp and paper industry. Related questions How do valence electrons affect chemical bonding? In a picture, the valence electrons are the ones in the outermost shell. Chlorine Facts and Science. Examples of Compounds with Chlorine Sodium Chloride This is sodium chloride , also known as table salt. Here, stable means that atom has not formed a ion yet. Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. Since the 3s if now full we'll move to the 3p where we'll place the remaining five electrons. Video: Chlorine Electron Configuration Notation.
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom.
Related questions How do valence electrons affect chemical bonding? Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. Additionally, they are very lightweight and are also used for many purposes in the healthcare industry, such as tubing. Video from: Noel Pauller. The atomic number is the number of protons present in the nucleus of an atom. The name trichloride means three chlorine atoms are involved. Food safety—Sanitizes food contact surfaces, and dilute chlorine bleach solutions are sometimes sprayed on fresh produce to reduce spoilage and the potential growth of pathogens. Chlorine then has the eight electrons in its outer shell to make it "happy". They are extremely useful thermoplastics that can replace rubber or metal pipes. Take a look at the dots around the atoms. Nitrogen Trichloride Nitrogen can combine with three chlorine atoms, forming Nitrogen trichloride , or NCl 3. A workhorse element with a wide range of important applications Below are some of the primary uses of chlorine chemistry: Swimming pool water—Kills germs in pool water to help control the spread of waterborne illnesses. Combines easily to form these very well-known compounds, among many others Sodium chloride NaCl —Known widely as common table salt, sodium chloride is an important component of the diets of both people and animals.

Yes, really. It was and with me. Let's discuss this question.