Hybridization of carbon in co2
In this article, we will delve into the intriguing world of chemistry to explore the hybridization of CO 2.
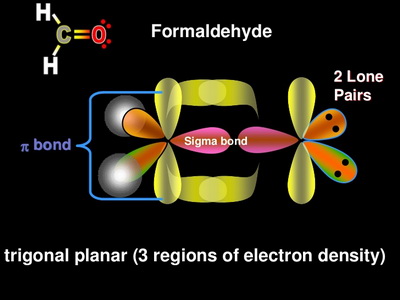
The carbon dioxide or CO2 has sp type hybridisation. This type of hybridisation occurs as an outcome of the carbon being bound to two different atoms. The atom of the carbon comprises 2 double bonds, i. However, this is not sufficient for creating bonds involving the oxygen. Therefore, one electron from the 2s orbital shifts from the 2s level to 2p level, which leads to the creation of 2 hybrid orbitals. These hybridised sp orbitals belonging to the carbon atoms extend beyond 2p orbitals that belong to the atoms of oxygen for creating two sigma bonds. A pi-bond is formed between the 2 leftover p electrons.
Hybridization of carbon in co2
To determine the hybridization of carbon dioxide, let us take the carbon atom first. The carbon atom has two double bonds, or two effective pairs exist in it. However, this is not enough to produce bonds with oxygen. So, then, one electron from 2s orbital moves from the 2s level to the 2p level that results in the formation of two hybrid orbitals. Now, these hybridized sp orbitals of carbon atoms overlap with two p orbitals of the oxygen atoms to produce 2 sigma bonds. They are used to form a pi bond as for the two remaining p electrons. In the carbon dioxide molecule, oxygen also hybridizes its orbitals to produce three sp2 hybrid orbitals. The p orbital in the oxygen atom remains unchanged and is primarily used to form a pi bond. However, out of these three sp hybrid orbitals, only one will be used to produce a bond with the carbon atom. Carbon dioxide has an sp hybridization type. This hybridization type occurs as a result of carbon being bound to the other two atoms.
Therefore, one electron from the 2s orbital shifts from the 2s level to 2p level, which leads to the creation of 2 hybrid orbitals. Lastly, if it is bonded with two atoms, then it is sp. Moreover, the hybridisation of each oxygen is then sp2.
We will learn about the hybridization of CO 2 on this page. Carbon dioxide basically has a sp hybridization type. This type of hybridization occurs as a result of carbon being bound to two other atoms. We can determine this by closely observing each atom of CO 2. In determining the hybridization of carbon dioxide, we will take the carbon atom first. The carbon atom has two effective pairs or two double bonds exist in it.
Carbon Dioxide is one of the best compounds to start with learning the concepts of Lewis structure and Molecular Geometry. This molecule can be a good start for beginners who want to learn the fundamentals of such concepts and want to know how to draw Lewis dot structures for other molecules as well. Although this gaseous molecule is known for its contribution to the greenhouse effect and global warming , one cannot deny that there are quite a lot of uses for this gas in several industries. To understand the physical properties, reactivity, and other chemical properties of a given compound, it is essential to know its molecular geometry. And to help you understand it, I have discussed the CO2 Lewis structure and its hybridization below.
Hybridization of carbon in co2
To determine the hybridization of carbon dioxide, let us take the carbon atom first. The carbon atom has two double bonds, or two effective pairs exist in it. However, this is not enough to produce bonds with oxygen. So, then, one electron from 2s orbital moves from the 2s level to the 2p level that results in the formation of two hybrid orbitals. Now, these hybridized sp orbitals of carbon atoms overlap with two p orbitals of the oxygen atoms to produce 2 sigma bonds. They are used to form a pi bond as for the two remaining p electrons.
Pelea davis vs garcia
Two of the 2p orbitals, for example, the 2px and 2pz, only hold one electron. JEE Marking Scheme. JEE Application Process. When we discuss the hybridization of carbon dioxide, we start with the central carbon atom. JEE Advanced Syllabus. Access more than. If it is bonded with three atoms, then it is sp2. Types of Impurity Defects. Get subscription. This hybridization type occurs as a result of carbon being bound to the other two atoms. Band Theory. Here, the carbon has only 1 bond, and it may Test Series.
In this article, we will delve into the intriguing world of chemistry to explore the hybridization of CO 2.
The carbon atom has two double bonds, or two effective pairs exist in it. Test your Knowledge on Carbon dioxide Q 5. What is the molecular geometry of CO2? Two of the 2p orbitals, for example, the 2px and 2pz, only hold one electron. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. The carbon dioxide bond angle is degrees. Types of Impurity Defects. Out of the three sp hybrid orbitals, only one is used to form a bond with the carbon atom. Carbon dioxide is an interesting molecule, with carbon at its core exhibiting sp hybridization. Access more than. Allotment of Examination Centre.

Bravo, this remarkable phrase is necessary just by the way
I think, that you are not right. I suggest it to discuss.