Hydrogen bromide lewis dot structure
Wiki User.
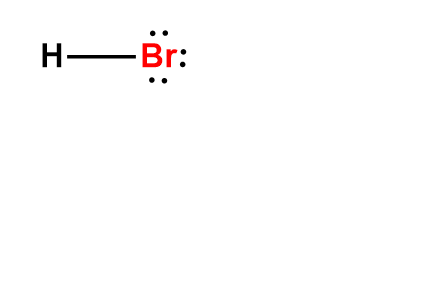
Wiki User. HBr has a dipole. HBr is a strong acid. HBr has an ionic bond. The Lewis dot structure for hydrogen bromide HBr consists of a single covalent bond between the hydrogen atom and the bromine atom. So, there is one single covalent bond in the Lewis dot structure of HBr.
Hydrogen bromide lewis dot structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydrogen bromide; hydrogen and bromine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of hydrogen and bromine atoms.
There is three lone pairs on bromine atom in HBr molecule. All Rights Reserved. Related questions.
.
The HBr Lewis Structure represents the arrangement of atoms and bonding electrons in a molecule of hydrogen bromide HBr. It showcases the connectivity between hydrogen H and bromine Br atoms, giving us a visual representation of their covalent bond. Determine the Total Valence Electrons. Arrange Atoms. The HBr molecule consists of only two atoms, so you can choose any atom as the central atom. Connect hydrogen and bromine with a single bond: H-Br. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Place the remaining electrons around the atoms. Hydrogen needs 2 electrons to complete its valence shell, while bromine needs 8.
Hydrogen bromide lewis dot structure
Transcript: Hi, this is Dr. Let's do the Lewis structure for HBr, hydrobromic acid. On the periodic table, Hydrogen is in group 1, so it has 1 valence electron, and Bromine is in group 7, sometimes called 17, it has 7 valence electrons; for a total of 8 valence electrons. We draw our H, we draw our Br, and we have 8 valence electrons.
Hilal cebeci erotik video
All Rights Reserved. These valence electrons determine the reactivity of the atom. Therefore, we can draw the skeleton of HBr easily. Also, remember that HBr is a molecule which does not have a charge. Find more answers Ask your question. HBr is a very easy lewis structure to draw due to its simplicity. Find more answers Ask your question. How many molecules are in 3. What kind of bond is HBr? There are no charges on hydrogen atom and HBr atom.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
Resources Leaderboard All Tags Unanswered. The Br atom then has 3 lone pairs placed around it. These valence electrons determine the reactivity of the atom. What is the symbolic notation for hydrogen? The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. What is the Lewis dot diagram for hydrogen and chlorine? However those all steps are mentioned and explained in detail in this tutorial for your knowledge. Still have questions? What is the Lewis dot diagram for lithium bromine carbon hydrogen silver oxygen iron oxygen potassium bromine and oxygine oxigine? When we draw a lewis structure, there are several steps to follow.

I can suggest to visit to you a site, with an information large quantity on a theme interesting you.
I am final, I am sorry, but this variant does not approach me.
I congratulate, this idea is necessary just by the way