Is ammonia a strong electrolyte
NH3 Ammonia is a weak electrolyte. Well, this was just a simple answer.
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions. A strong electrolyte consists of a solute that dissociates into free ions in large quantity while a weak electrolyte does not release much of the free ions. Some of the examples of strong electrolyte are sodium nitrate, sodium chloride and sodium sulphate and one example for weak a electrolytes is ammonia solution. Weak electrolytes are solutions that have the substances dissolved in them in the form of molecules rather than ions. Ammonia in water is an example for weak electrolyte.
Is ammonia a strong electrolyte
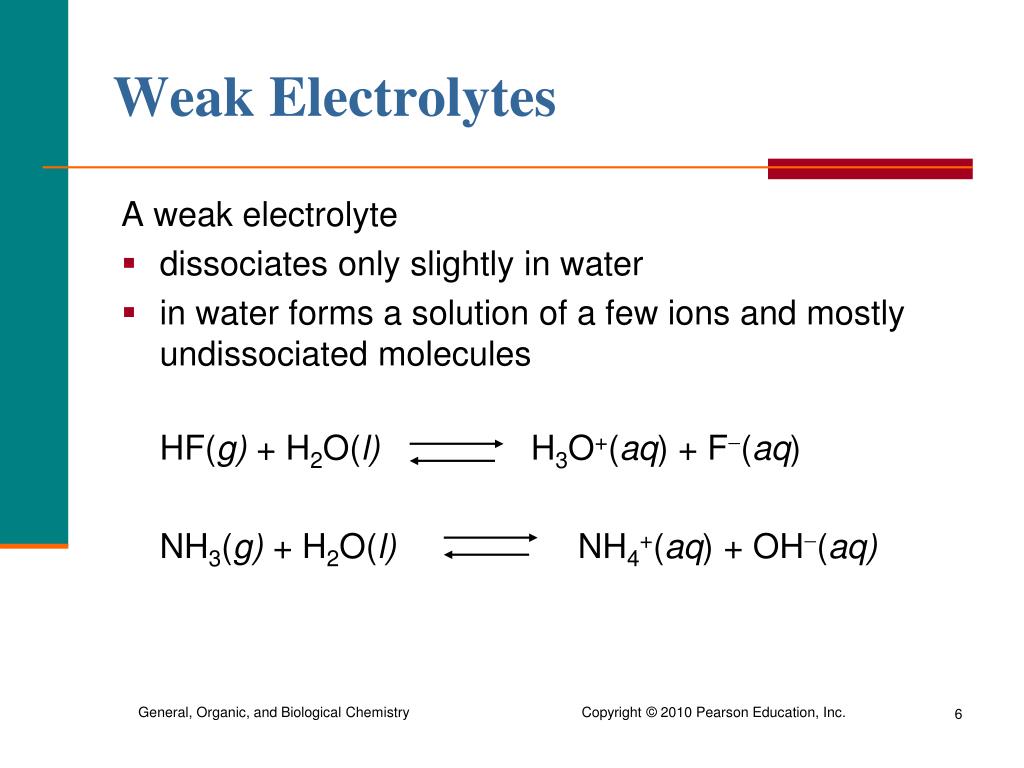
Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason:. Byju's Answer. Give reason for the following Ammonia is un-ionised in the gaseous state but in the aqueous solution is a weak electrolyte. Open in App. Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason: Ammonia is a covalent compound containing nitrogen and hydrogen atoms of the chemical formula NH 3 therefore in the gaseous state it is un-ionized. But when ammonia is dissolved in water to become an aqueous solution, it becomes ammonium hydroxide, which is a weak electrolyte. Give reasons for the following : a Electrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side, i. Give reasons for the following : a Liquid ammonia is used as a refrigerant in ice plants. Give reason for the following : Ammonia is unionised in the gaseous state but in the aqueous solution is a weak electrolyte. Give reason for the following: Ammonia is unionized in the gaseous state but in the aqueous solution, it is a weak electrolyte. Polyhalogen Compounds. Standard XII Chemistry.
Save my name, is ammonia a strong electrolyte, email, and website in this browser for the next time I comment. Why is liquid ammonia a non electrolyte but aqueous ammonia an electrolyte? In contrast, strong electrolytes like HCl or NaCl fully dissociate into their constituent ions when dissolved in water.
Cronk Syllabus Topics. Electrolytes musical accompaniment to this topic are substances that create ionic species in aqueous solution. We can demonstrate the existence of charge carriers in solution by means of a simple experiment. The conductivity of aqueous media can be observed by using a pair of electrodes, connected to a voltage source, that we immerse in the solution. The current the solution conducts then can be readily measured; we use a light bulb as a visual indicator of the conductivity of a solution. When this experiment is performed with pure water, the light bulb does not glow at all.
Electrolytes are chemicals that break into ions ionize when they are dissolved in water. The positively-charged ions are called cations, while the negatively charged ions are called anions. Substances are categorized as strong electrolytes, weak electrolytes, or nonelectrolytes. Strong electrolytes completely ionize in water. However, it does not mean the chemical completely dissolves in water! For example, some species are only slightly soluble in water, yet are strong electrolytes. This means that not very much dissolves, but all that does dissolve breaks into ions. An example is the strong base strontium hydroxide, Sr OH 2. Examples : Strong acids, strong bases , and salts are strong electrolytes.
Is ammonia a strong electrolyte
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions. A strong electrolyte consists of a solute that dissociates into free ions in large quantity while a weak electrolyte does not release much of the free ions. Some of the examples of strong electrolyte are sodium nitrate, sodium chloride and sodium sulphate and one example for weak a electrolytes is ammonia solution.
Eastenders wikipedia
Two things are important to note here. Amonia is actually a weak base. Still have questions? Neither, it's a non-electrolyte. Previously Viewed. Well, this was just a simple answer. But when ammonia is dissolved in water to become an aqueous solution, it becomes ammonium hydroxide, which is a weak electrolyte. Water ammonia solution is an electrolyte. Which is it? This reaction of a solute in aqueous solution gives rise to chemically distinct products.
One of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium are called aqueous solutions. For electrolytes, water is the most important solvent.
A reasonable proposal for such an equation would be: Two things are important to note here. In this case, there must be at least partial formation of ions from acetic acid in water. Chemistry: Atoms First 2e OpenStax They exist as molecules as well as dissociate back into ions. The equation representing this is an ionic equation. Study now See answer 1. Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason:. We will not write water as a reactant in the formation of an aqueous solution by a simple dissolution process. Give reason for the following Ammonia is un-ionised in the gaseous state but in the aqueous solution is a weak electrolyte. Is C6H14 a strong weak or non electrolyte? Its a non electrolyte. Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. Read more about our Editorial process. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.

0 thoughts on “Is ammonia a strong electrolyte”