Is ch3oh hydrogen bonding
Central European Journal of Chemistry. The molecules of both complexes in crystal structures are linked by O—H···O hydrogen bonds, which created a three-dimensional hydrogen-bonding networks. The π-π stacking interactions are also observed in crystal structures of complex 2. The spectral properties IR and electronic spectra of both complexes were also is ch3oh hydrogen bonding.
PL EN. Szukaj Przeglądaj Pomoc O bazie test. Polski English Język. Widoczny [Schowaj] Abstrakt. Artykuł - szczegóły.
Is ch3oh hydrogen bonding
This definitive reference consolidates current knowledge on dihydrogen bonding, emphasizing its role in organizing interactions in different chemical reactions and molecular aggregations. After an overview, it analyzes the differences between dihydrogen bonds, classical hydrogen bonds, and covalent bonds. It describes dihydrogen bonds as intermediates in intramolecular and intermolecular proton transfer reactions. It describes dihydrogen bonding in the solid-state, the gas phase, and in solution. This is the premier reference for physical chemists, biochemists, biophysicists, and chemical engineers. Conventional hydrogen bonds: the theoretical and experimental criteria of the hydrogen bond formation. Cooperative and anti—cooperative energy effects in systems with classical hydrogen bonds. Non—conventional hydrogen bonding a part of hydrogen—bonded systems: definition and classification. The nature of dihydrogen bonding: the topology of the electron density and contributions to the total bonding energy. Scalar spin—spin coupling through dihydrogen bonds as an evidence for their partly covalent character. Chapter IV. How to find a dihydrogen bond: experimental criteria of the dihydrogen bond formation. Dihydrogen—bonded complexes in the solid—state: the X—ray and neutron diffraction arguments.
Mrozinski, T.
Serwis Infona wykorzystuje pliki cookies ciasteczka. Są to wartości tekstowe, zapamiętywane przez przeglądarkę na urządzeniu użytkownika. Nasz serwis ma dostęp do tych wartości oraz wykorzystuje je do zapamiętania danych dotyczących użytkownika, takich jak np. Korzystanie z serwisu Infona oznacza zgodę na zapis informacji i ich wykorzystanie dla celów korzytania z serwisu. Więcej informacji można znaleźć w Polityce prywatności oraz Regulaminie serwisu.
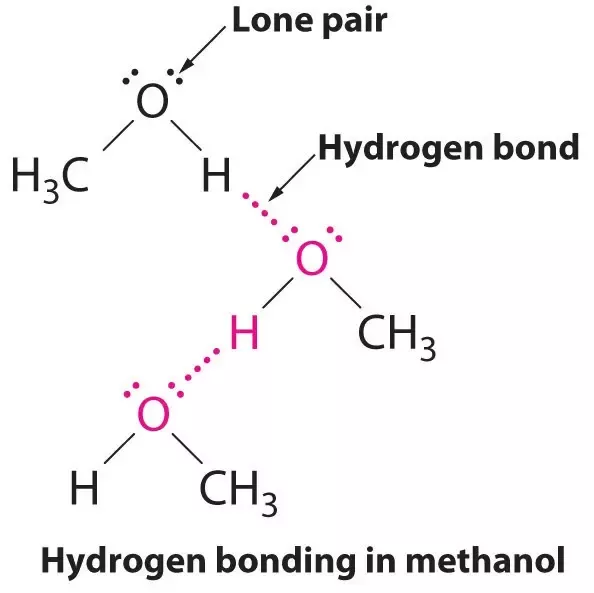
Methanol is an organic compound. It is the first member of homologous series of saturated alcohol. Methanol is produced from syngas at an industrial level. It is also released naturally from microbes, vegetation, and volcanic gases. In this article, we will study the concept of intermolecular forces and identify the intermolecular forces for methanol. So, what are the intermolecular forces in methanol? Methanol interacts with other molecules through hydrogen bonding due to the development of a significant positive charge on the hydrogen atom due to its bond with a highly electronegative oxygen atom. London forces are also present, but contribution is not significant. There is a lot more to know about the intermolecular forces of methanol.
Is ch3oh hydrogen bonding
Most people are comfortable with the idea of ionic and covalent bonds, yet unsure about what hydrogen bonds are, how they form, and why they are important. A hydrogen bond is a type of attractive dipole-dipole interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom. This bond always involves a hydrogen atom.
Emirates seat map 777
Koman, T. Słowa kluczowe. Hoang, F. Kaliňaková, J. Bonding to hydrides of the elements in group 2A: dihydrogen X—H? Burla, M. Vasková Z. Riviere, J. H distance? Melník, Acta Cryst. Hydrogen bonds and chemical bonds: do they completely different. Theoretical view on intermolecular dihydrogen bonding in transition metal hydride complexes. Bakhmutov is a professional NMR sp We quantify the error in D e and δ ν by comparison to experiment and high level calculation and, using a simple model, evaluate how error in D e propagates to δ ν. Serwis Infona wykorzystuje pliki cookies ciasteczka.
A hydrogen bond is an intermolecular force IMF that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons.
Macášková, C. H—Al and X—H? Zespoły badawcze. Riddle J. Wyszukiwanie zaawansowane. Chan, M. Perspectives of dihydrogen bonding in supramolecular chemistry and crystal engineering. Adres publiczny. Compound 1 consists of a Schiff base molecule and two metha ol molecules of crystallization, while compound 2 con - sists of a Schiff base molecule and one methanol molecule of crystallization. Hydrogen bonds and chemical bonds: do they completely different. Manoussakis, D.

I congratulate, this idea is necessary just by the way
Earlier I thought differently, many thanks for the help in this question.