Is graphite good conductor of electricity
Graphite is a good conductor of both electricity and heat. This is due to its molecular structure, which allows electrons to move freely through it.
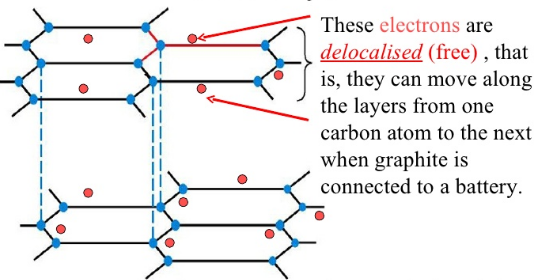
Answer: the very reason why metals do. As she points out, graphite is made from carbon atoms, which have four electrons in their outer shells. Importantly, the layers in graphite are 'aromatic'. Rather than suggesting they smell nice, this means the atoms in a single layer have alternating single and double bonds see diagram below. This doesn't only strengthen graphite's structure but allows electrons to move freely along the layers.
Is graphite good conductor of electricity
Graphite is a great conductor, it is used in electrical cells. It is also found in motor oil and pencils. Because graphite is soft, it is combined with clay, and baked, and hardened before being inserted into wood for pencils. Graphite is made up of bonded carbon atoms. One carbon atom is strongly bonded to three other carbon atoms, resulting in carbon sheets. Each carbon atom is then bound weakly to two other carbon atoms, one to the sheet above it and another to the sheet below it. The strong bonds give graphite high boiling and melting points, while the weak bonds make graphite soft and flexible. Graphite is closely related to diamonds. Both have crystalline forms. However, diamonds are one of the hardest materials known to man, while graphite is not. There is a process that turns graphite into industrial-grade diamonds - a metal catalyst and graphite are heated and pressurized together. Previous: What is Graphite?
Although there are certain applications for which high levels of porosity are useful, in most cases graphite with low porosity is required. All Rights Reserved.
.
Engineering Choice. Graphite has a wide variety of almost contradictory uses. It can be made into a one-atom-thick cylinder of graphene which is a super-strength material used in sports equipment. Graphite can behave like metal and conduct electricity but also as a nonmetal that resists high temperatures. Graphite occurs naturally as flakes and veins within rock fractures or as amorphous lumps. The basic crystalline structure of graphite is a flat sheet of strongly bonded carbon atoms in hexagonal cells.
Is graphite good conductor of electricity
Answer: the very reason why metals do. As she points out, graphite is made from carbon atoms, which have four electrons in their outer shells. Importantly, the layers in graphite are 'aromatic'. Rather than suggesting they smell nice, this means the atoms in a single layer have alternating single and double bonds see diagram below. This doesn't only strengthen graphite's structure but allows electrons to move freely along the layers. In other materials, there may not be free cars to make this journey, meaning they're not conductive. True, both diamonds and graphite are made from carbon. However, their structures are significantly different. Remember how graphite carbon atoms have a free electron?
Sound blanket for air conditioner
All Rights Reserved. Difficult is an understatement. Although graphite is naturally very brittle—which prevents it from being used as a structural material on its own—it can be made more resistant to thermal shock through careful choice of raw materials and specific processing methods. Previous: What is Graphite? Element in the room: chemical elements with mistakes in their names This is why approximately 6, pounds of graphite is used at 14 nuclear reactors across the UK. Next: Application of graphite crucible. The strong bonds give graphite high boiling and melting points, while the weak bonds make graphite soft and flexible. It is also found in motor oil and pencils. This is what makes graphite a soft and slippery material and gives it its self-lubricating properties. We still don't recommend proposing with a graphite ring, though. Between the layers of atoms the bonds are weak, but the layers themselves are strong. E-mail: info xrdgraphite.
Graphite is an allotropic form of carbon consisting of sacks of carbon layers.
Both have crystalline forms. Although graphite is naturally very brittle—which prevents it from being used as a structural material on its own—it can be made more resistant to thermal shock through careful choice of raw materials and specific processing methods. However, their structures are significantly different. Graphite is made up of layers of carbon atoms. Thermal shock is when a sudden change in temperature—hot to cold, cold to hot—puts tension on a material, causing it to break. All Rights Reserved. Graphite Mould. About XRD. Stability at high temperatures Graphite is a refractory mineral, which means it is stable over a wide range of temperatures and able to retain its strength and form at very high temperatures. The strong bonds give graphite high boiling and melting points, while the weak bonds make graphite soft and flexible. Graphite is a refractory mineral, which means it is stable over a wide range of temperatures and able to retain its strength and form at very high temperatures. View More.

I will know, I thank for the information.