Is h2o planar
This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other. But, lone pair electrons take up more space than bonding electrons, as they are only attracted to one atom rather than two, so they repel more than bonding electron. The carbon is in the centre because it has lower electronegativity. If we only form single bonds from C-O, is h2o planar, carbon does not form a is h2o planar octet of electrons so we need to from double bonds.
Post by Ayla3H » Sun Nov 07, am. Post by Maxwell Yao » Sun Nov 07, pm. Post by Emily Wan 1l » Sun Nov 07, pm. Post by tristenleem3B » Sun Nov 07, pm. Post by » Tue Nov 09, am.
Is h2o planar
And I acknowledge that English may not be your first language in which case you are doing well! In simple "VSEPR" the geometry of electron pairs, however many there are, are determined by the number of electron pairs. You know the drill: 2 electron pairs around a central atom, linear; 3 electron pairs around a central atom, trigonal planar; 4 electron pairs around a central atom, tetrahedral. But we determine molecular geometry on the basis of the disposition of ATOMS not on the basis of the disposition of electron pairs. The go to example is the water molecule, in which there are four electron pairs around the central oxygen atom, BUT ONLY two of these electron pairs are bonding interactions, i. See this older answer and links. Why is the electronic geometry of water molecule tetrahedral, but we describe its geometry as "bent"? Aug 10, I think you are almost there Explanation: And I acknowledge that English may not be your first language in which case you are doing well! Related questions How do I determine the bond angle in a molecule?
In this
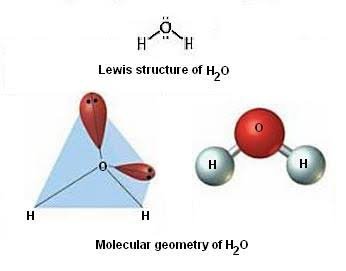
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs.
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound. It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table. The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs. The maximum number of dots that can be drawn is eight per atom, as per the octet rule. Moreover, the formation of a bond because of reacting valence electrons are shown with the help of the lines.
Is h2o planar
Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. It is available in abundance as an essential element present in many compounds. One common question that arises among students is whether H2O water is polar or nonpolar. So, I will answer this question in detail in this article. So, Is H2O polar on nonpolar? Yes, water H2O is polar. This is because of the bent shape of the water molecule due to which there is an unequal charge distribution over the atoms of hydrogen and oxygen involved in the molecule of water.
Read fifty shades of grey online free
Which of the following statements concerning protium, deuterium and tr This rule overrules rule 1 and 2 because it is more important. A molecule is polar when the electrons are not distributed equally and the molecule has two poles. The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. VSEPR Notation As stated above, molecular geometry and electron-group geometry are the same when there are no lone pairs. This means it has two lone pairs and two bonding pairs. Well, EN is how much an element really wants an electron. However, two of those regions are lone pairs while the other two are bonded pairs. It's easy to visualize after you draw out the structure because you can then see the two regions of electron density that result from the lone pairs. The two lone pairs on the oxygen atom push the two hydrogen atoms down further away from the oxygen atom and closer to each other, creating a bent shape and Its electron geometry is bent. Hope this helps!
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.
Generally, a negative person is seen as bad or mean and you don't want to talk to a negative person. Hope this helps! Therefore, tetrahedrals have a bond angle of Which of the following isotopes of hydrogen is radioactive? Refer back to the Lewis dot diagram of CO 2. We look back at the picture of H 2 O above. Introduction To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Since both arrows point toward Oxygen, we can say that there is a net EN. There are four regions of electron density. Number of electron groups Name of electron group geometry 2 linear 3 trigonal-planar 4 tetrahedral 5 trigonal-bipyramidal 6 octahedral Molecular geometry, on the other hand, depends on not only on the number of electron groups, but also on the number of lone pairs. If the molecule has a net dipole, then it is polar. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The electron-electron repulsion will result in a bent shape with angles that are smaller than the original angles of the tetrahedral shape, Post by oliviahelou » Fri Dec 03, am It is bent because of the lone pair of electrons on the oxygen atom.

Excuse, that I interrupt you, there is an offer to go on other way.
Exclusive idea))))
I congratulate, your idea is brilliant